| << Chapter < Page | Chapter >> Page > |
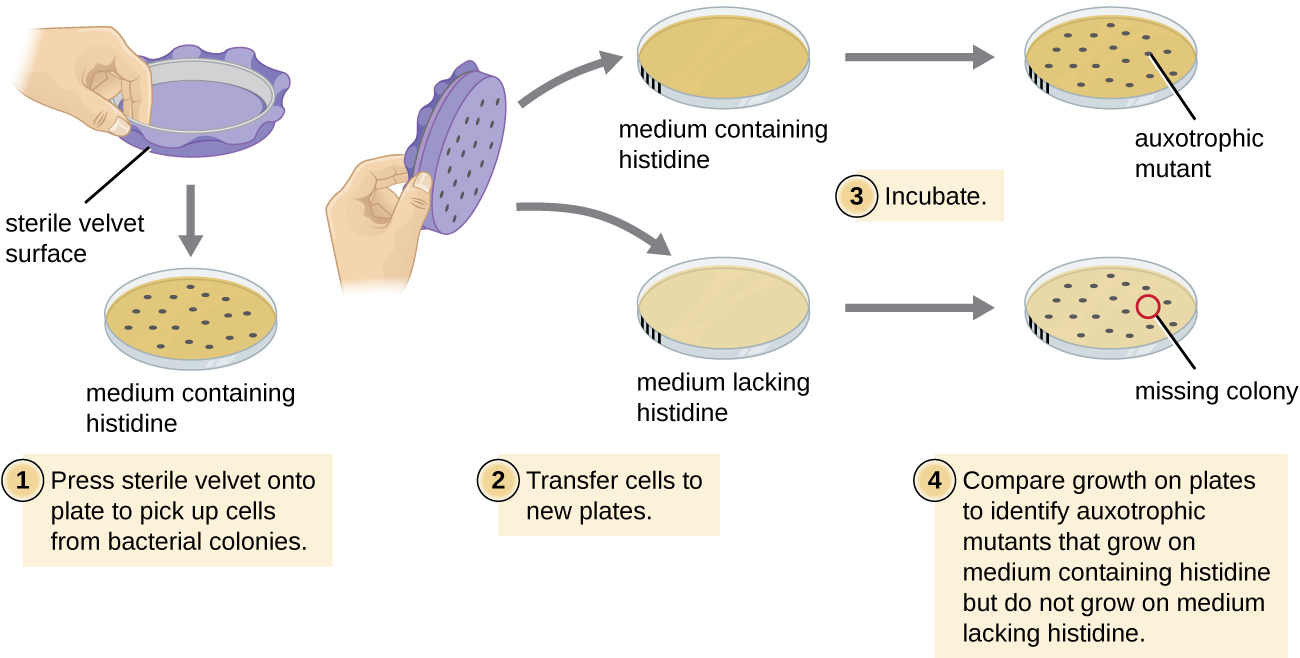
One common technique used to identify bacterial mutants is called replica plating . This technique is used to detect nutritional mutants, called auxotroph s, which have a mutation in a gene encoding an enzyme in the biosynthesis pathway of a specific nutrient, such as an amino acid. As a result, whereas wild-type cells retain the ability to grow normally on a medium lacking the specific nutrient, auxotrophs are unable to grow on such a medium. During replica plating ( [link] ), a population of bacterial cells is mutagenized and then plated as individual cells on a complex nutritionally complete plate and allowed to grow into colonies. Cells from these colonies are removed from this master plate, often using sterile velvet. This velvet, containing cells, is then pressed in the same orientation onto plates of various media. At least one plate should also be nutritionally complete to ensure that cells are being properly transferred between the plates. The other plates lack specific nutrients, allowing the researcher to discover various auxotrophic mutants unable to produce specific nutrients. Cells from the corresponding colony on the nutritionally complete plate can be used to recover the mutant for further study.
The Ames test , developed by Bruce Ames (1928–) in the 1970s, is a method that uses bacteria for rapid, inexpensive screening of the carcinogenic potential of new chemical compounds. The test measures the mutation rate associated with exposure to the compound, which, if elevated, may indicate that exposure to this compound is associated with greater cancer risk. The Ames test uses as the test organism a strain of Salmonella typhimurium that is a histidine auxotroph, unable to synthesize its own histidine because of a mutation in an essential gene required for its synthesis. After exposure to a potential mutagen, these bacteria are plated onto a medium lacking histidine, and the number of mutants regaining the ability to synthesize histidine is recorded and compared with the number of such mutants that arise in the absence of the potential mutagen ( [link] ). Chemicals that are more mutagenic will bring about more mutants with restored histidine synthesis in the Ames test. Because many chemicals are not directly mutagenic but are metabolized to mutagenic forms by liver enzymes, rat liver extract is commonly included at the start of this experiment to mimic liver metabolism. After the Ames test is conducted, compounds identified as mutagenic are further tested for their potential carcinogenic properties by using other models, including animal models like mice and rats.

Notification Switch
Would you like to follow the 'Microbiology' conversation and receive update notifications?