| << Chapter < Page | Chapter >> Page > |

Click this link to see an animation of how the cholera toxin functions.
Click this link to see an animation of how the botulinum toxin functions.
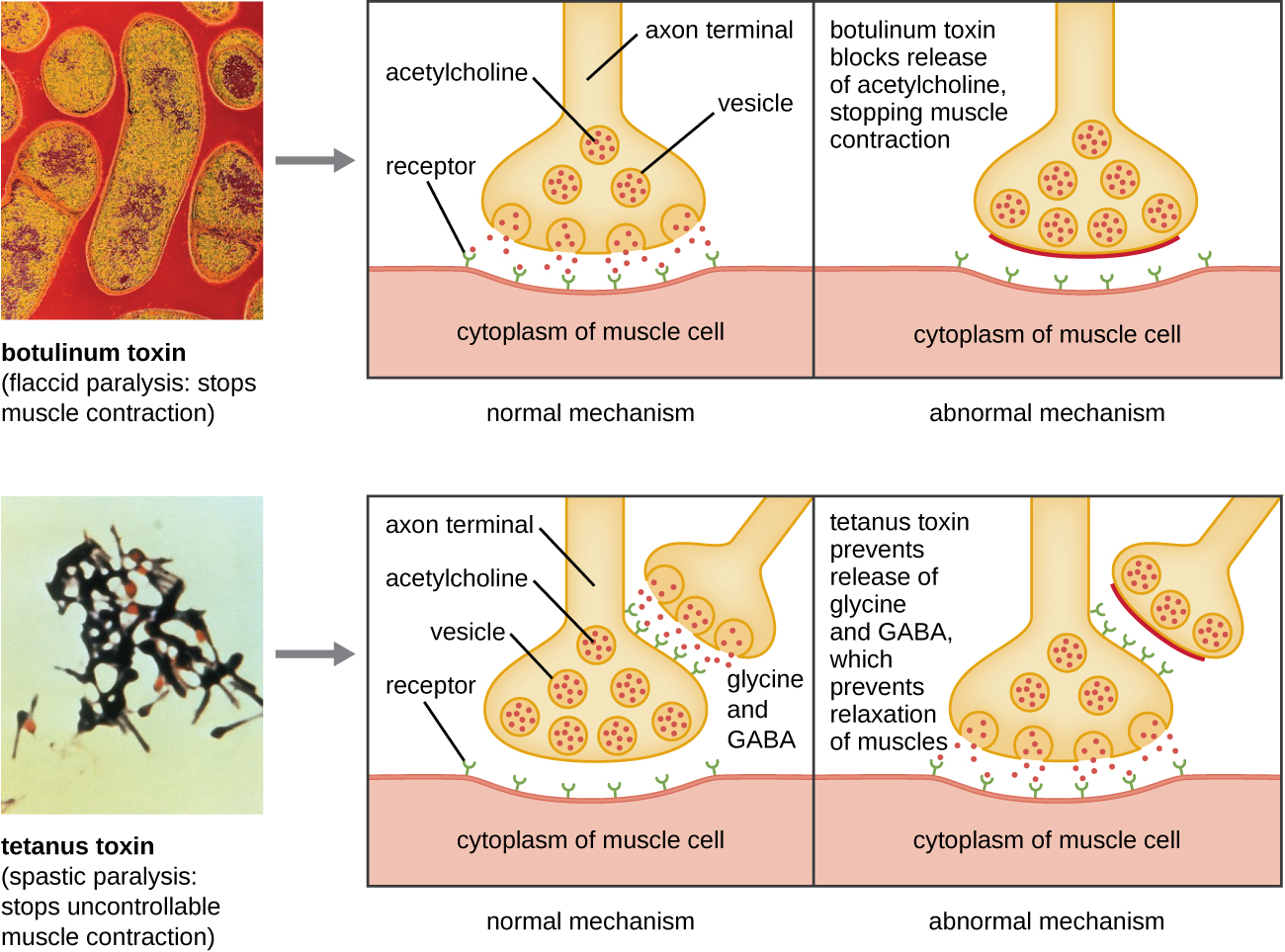
Another neurotoxin is tetanus toxin , which is produced by the gram-positive bacterium Clostridium tetani . This toxin also has a light A subunit and heavy protein chain B subunit. Unlike botulinum toxin, tetanus toxin binds to inhibitory interneurons, which are responsible for release of the inhibitory neurotransmitters glycine and gamma-aminobutyric acid (GABA). Normally, these neurotransmitters bind to neurons at the neuromuscular junction, resulting in the inhibition of acetylcholine release. Tetanus toxin inhibits the release of glycine and GABA from the interneuron, resulting in permanent muscle contraction. The first symptom is typically stiffness of the jaw (lockjaw). Violent muscle spasms in other parts of the body follow, typically culminating with respiratory failure and death. [link] shows the actions of both botulinum and tetanus toxins.
Membrane-disrupting toxins affect cell membrane function either by forming pores or by disrupting the phospholipid bilayer in host cell membranes. Two types of membrane-disrupting exotoxins are hemolysin s and leukocidins , which form pores in cell membranes, causing leakage of the cytoplasmic contents and cell lysis. These toxins were originally thought to target red blood cells (erythrocytes) and white blood cells (leukocytes), respectively, but we now know they can affect other cells as well. The gram-positive bacterium Streptococcus pyogenes produces streptolysins , water-soluble hemolysins that bind to the cholesterol moieties in the host cell membrane to form a pore. The two types of streptolysins, O and S, are categorized by their ability to cause hemolysis in erythrocytes in the absence or presence of oxygen. Streptolysin O is not active in the presence of oxygen, whereas streptolysin S is active in the presence of oxygen. Other important pore-forming membrane-disrupting toxins include alpha toxin of Staphylococcus aureus and pneumolysin of Streptococcus pneumoniae .
Bacterial phospholipases are membrane-disrupting toxin s that degrade the phospholipid bilayer of cell membranes rather than forming pores. We have already discussed the phospholipases associated with B. anthracis, L. pneumophila, and Rickettsia species that enable these bacteria to effect the lysis of phagosomes. These same phospholipases are also hemolysins. Other phospholipases that function as hemolysins include the alpha toxin of Clostridium perfringens , phospholipase C of P. aeruginosa , and beta toxin of Staphylococcus aureus .
Some strains of S. aureus also produce a leukocidin called Panton-Valentine leukocidin (PVL) . PVL consists of two subunits, S and F. The S component acts like the B subunit of an A-B exotoxin in that it binds to glycolipids on the outer plasma membrane of animal cells. The F-component acts like the A subunit of an A-B exotoxin and carries the enzymatic activity. The toxin inserts and assembles into a pore in the membrane. Genes that encode PVL are more frequently present in S. aureus strains that cause skin infections and pneumonia. V. Meka. “Panton-Valentine Leukocidin.” http://www.antimicrobe.org/h04c.files/history/PVL-S-aureus.asp PVL promotes skin infections by causing edema, erythema (reddening of the skin due to blood vessel dilation), and skin necrosis. PVL has also been shown to cause necrotizing pneumonia. PVL promotes pro-inflammatory and cytotoxic effects on alveolar leukocytes. This results in the release of enzymes from the leukocytes, which, in turn, cause damage to lung tissue.

Notification Switch
Would you like to follow the 'Microbiology' conversation and receive update notifications?