| << Chapter < Page | Chapter >> Page > |
Alkyl lithium compounds are either low melting solids or liquids, and often with high volatility (depending on the substituent) due to the covalent nature of the bonding. They are soluble in aliphatics, aromatics, and ethers. However, while the reaction with ethers is generally slow, [link] , alkyl lithium compounds can polymerize tetrahydrofuran (THF).
Organolithium compounds react rapidly with air and water (both vapor and liquid). The reaction with water is the basis of the Gillman double titration method for determining the concentration of organolithium reagents in solution.
The structure of organolithium compounds is dominated by their highly oligomeric nature as a result of 3-center 2-electron bridging bonds. In all cases the extent of oligomerization is dependant on the identity of the alkyl (or aryl) group. The alkyl-bridged bond is similar to those found for beryllium and aluminum compounds.
In the vapor phase any particular organolithium derivative show a range of oligomeric structures. For example, the mass spectrum of EtLi shows ions associated with both tetramers (e.g., [Et 3 Li 4 ] + ) and hexamers (e.g., [Et 5 Li 6 ] + ). The structures of the different oligomers have been predicted by molecular orbital calculations ( [link] ).
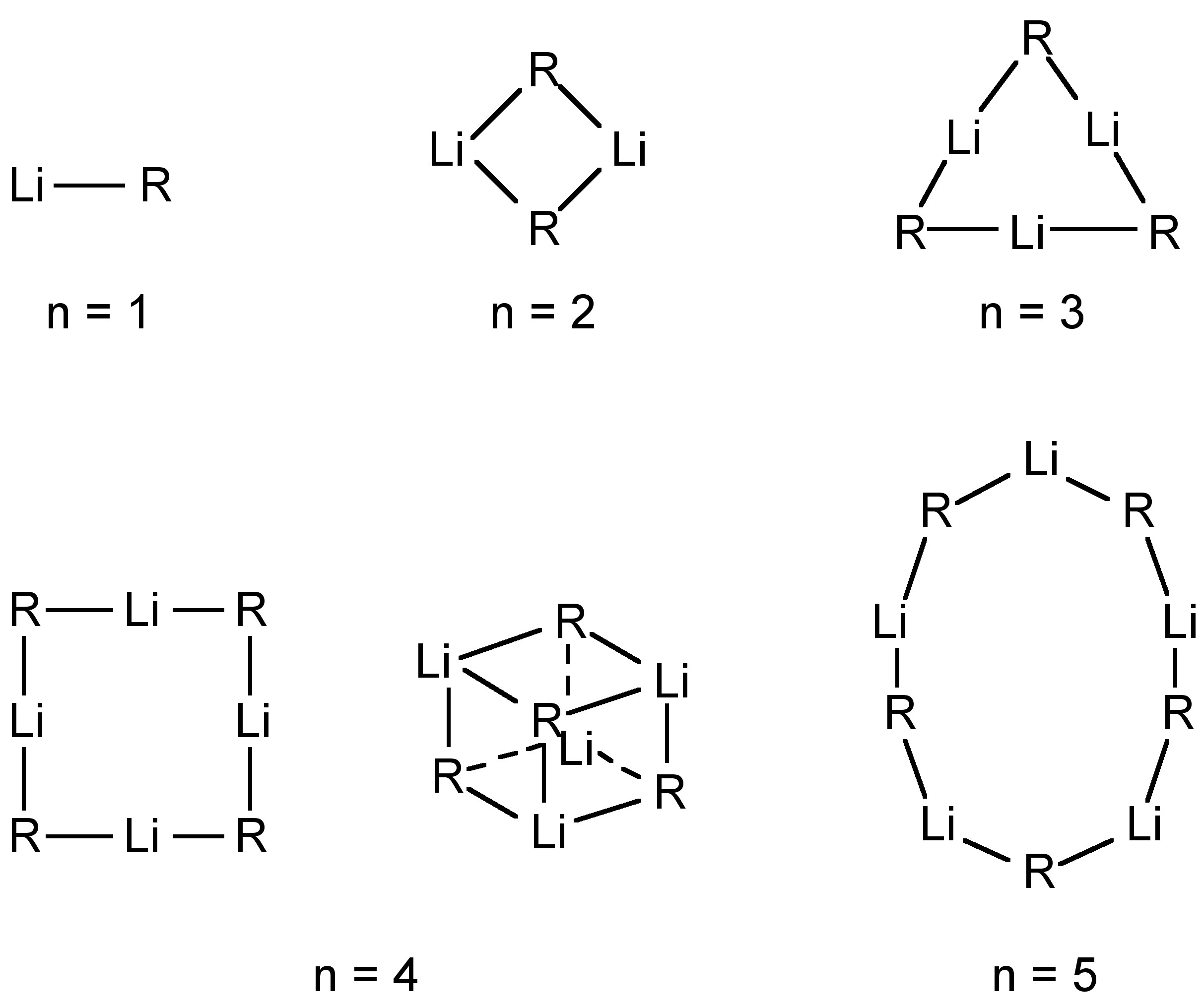
Solution molecular weight measurements indicate the oligomerization is present (in the absence of a coordinating ligand such as Et 2 O or an amine). The extent of oligomerization depends on the steric bulk of the alkyl group ( [link] ). Oligomerization and solution structures have also been investigated by 7 Li and 13 C NMR spectroscopy.
| R | [RLi] n | R | [RLi] n |
| Me | 4 | Et | 6 |
| n Bu | 6 | t Bu | 4 |
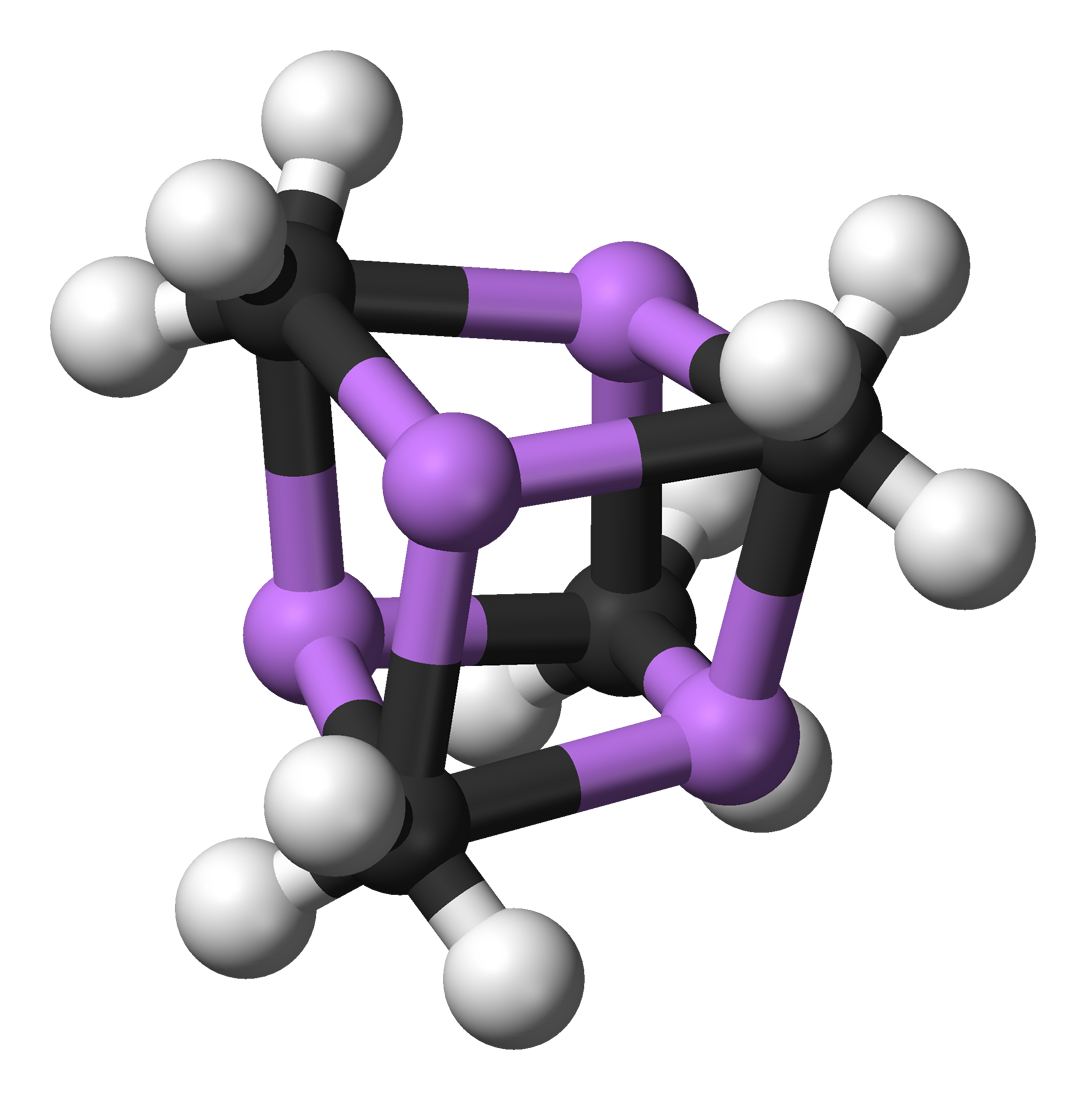
There are a large number of X-ray crystallographically determined structures for organolithium derivatives. The archetypal example is MeLi, which exists as a tetramer in the solid state ( [link] ). The lithium atoms are arranged as a tetrahedron and the carbon atoms are positioned on the center of the facial planes, i.e., the carbon is equidistant from each of the lithium atoms. In contrast, EtLi has a similar tetrahedral structure, but the α-carbon of the ethyl groups are asymmetrically arranged such that it is closer to one lithium atom than the other two.
It is possible to prepare monomeric organolithium compounds by the addition of amines, especially chelate ligands such as ethylenediamine (en) and tetramethylethylenediamine (TMED). The reactivity of RLi is increased dramatically by the addition of such Lewis bases. For example, PhCH 2 Li shows an increased reactivity of 10 4 with the addition of TMED.
The bonding in organolithium compounds is difficult to describe:
However, the overall description of the bonding in RLi is that of a covalent interaction with significant polar (ionic) character, i.e., M δ + -C δ - .
Organolithium compounds perform many of the reactions commonly observed for Grignard reagents. However, lithium reagents are generally more reactive than their Grignard analogs.
Organolithium compounds react with water to give the hydrocarbon and lithium hydroxide, [link] . Lithium alkyls also react with other hydroxylic compounds such as alcohols and carboxylic acids, [link] .
One important use of the hydrolysis reaction is specifically deuteration, [link] .
Organolithium compounds react with organic carbonyls (aldehydes, ketones, and esters) to yield the alcohol on hydrolysis, [link] . This synthetic route is particularly useful since lithium reagents are far more reactive than the analogous Grignard, allowing reactions to be carried out at lower temperatures and minimizing enolization side reactions.
The high reactivity of alkyl lithium compounds means that they react with carboxylic acids to yield the ketone rather than the lithium carboxylate.

Organolithium compounds generally react with α,β-unsaturated ketones to give the 1,2-addition product, [link] . However, lithium dialkylcuprates, which are formed from the alkyl lithium and copper(I) iodide, [link] , add exclusively by the 1,4-addition, [link] .
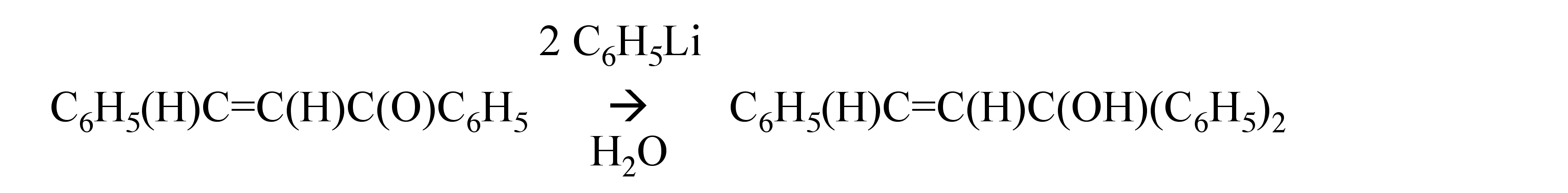
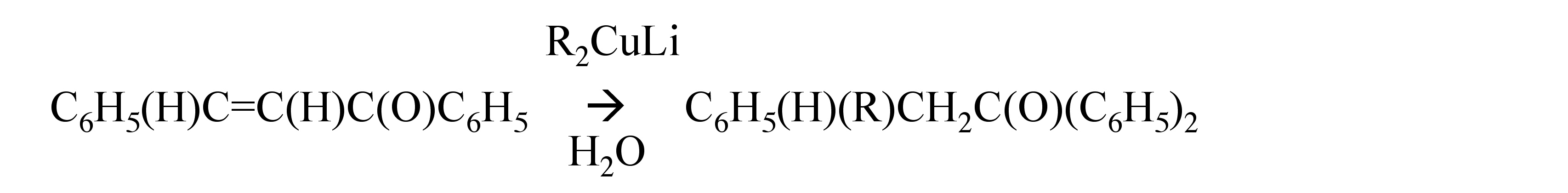
One of the most useful methods of preparing organometallic compounds is the exchange reaction of one organometallic compound with a salt of a different metal, [link] . This is an equilibrium process, whose equilibrium constant is defined by the reduction potential of both metals. In general the reaction will proceed so that the more electropositive metal will form the more ionic salt (usually chloride).
Lithium reagents may be used to prepare a wide range of organometallic compounds.

Notification Switch
Would you like to follow the 'Chemistry of the main group elements' conversation and receive update notifications?