| << Chapter < Page | Chapter >> Page > |
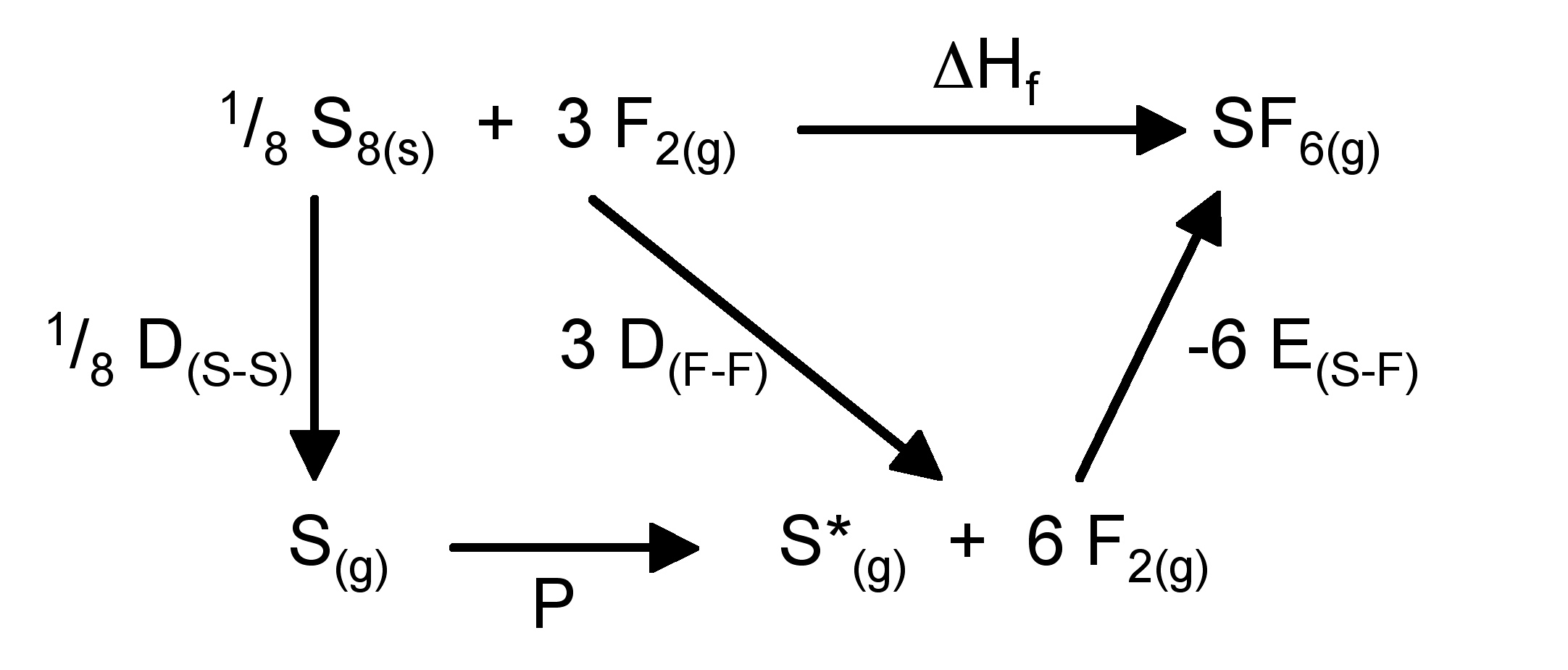
Sulfur hexafluoride (SF 6 ) is a gas at standard temperature and pressure (25 °C, 1 atm). The most common synthesis involves the direct reaction of sulfur with fluorine yields SF 6 .
It should be noted that while SF 6 is highly stable, SCl 6 is not formed. The explanation of this difference may be explained by a consideration of the Born-Haber cycle shown in [link] . A similar cycle may be calculated for SCl 6 ; however, a combination of a higher dissociation energy for Cl 2 and a lower S-Cl bond energy ( [link] ) provide the rational for why SCl 6 is not formed.
| Bond dissociation energy | kJ/mol | Bond energy | kJ/mol |
| D (F-F) | 158 | E (S-F) | 362 |
| D (Cl-Cl) | 262 | E (S-Cl) | 235 |
The S-F bond length (1.56 Å) is very short and consistent with π-bonding in addition to σ-bonding. Like SiF 6 2- , SF 6 is an example of a hypervalent molecule ( [link] ).
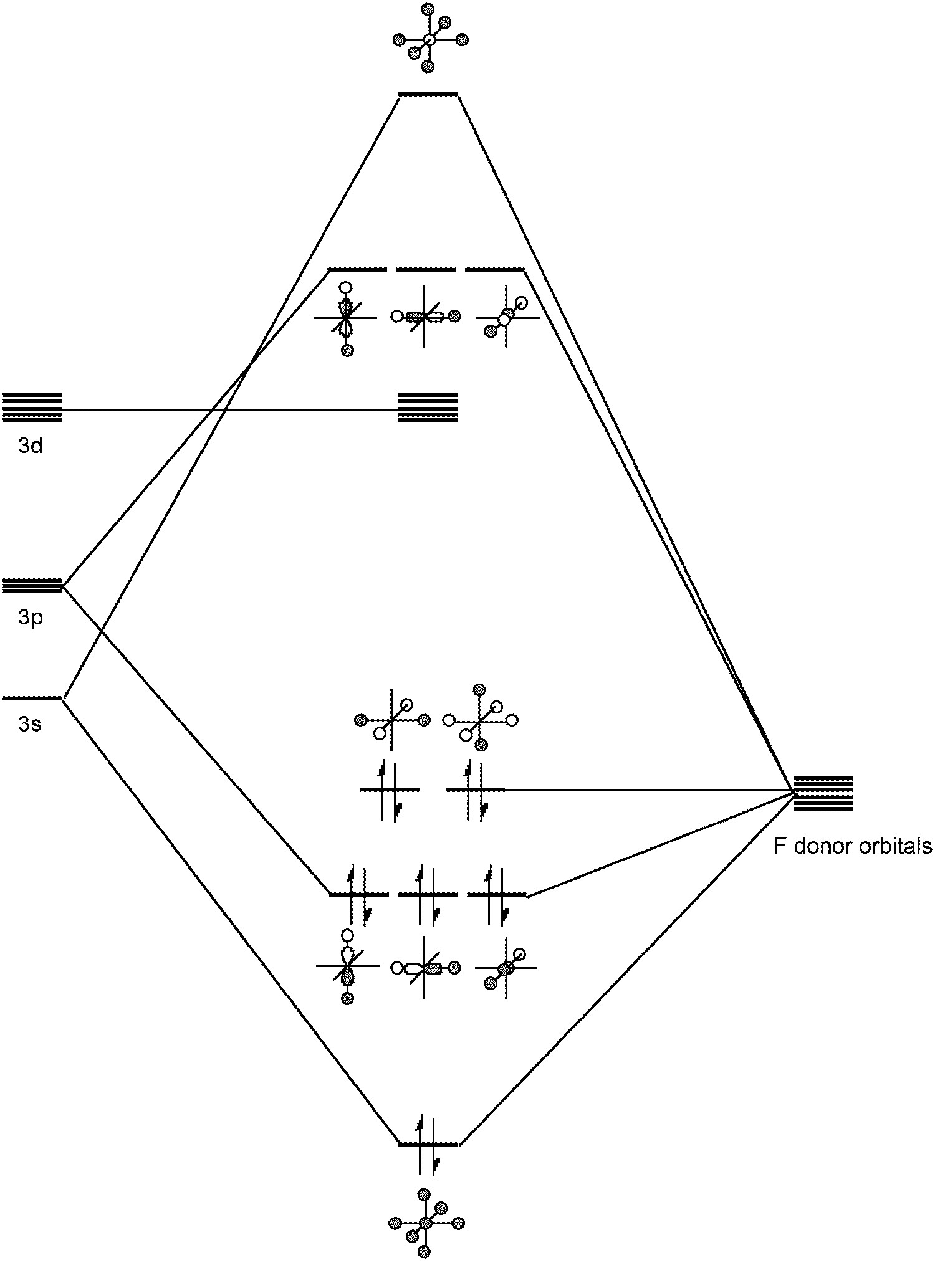
Sulfur hexafluoride is an unreactive, non toxic compound. Its inert nature provides one of its applications, as a spark suppressor. The hexafluoride is generally resistant to chemical attack, e.g., no reaction is observed with potassium hydroxide (KOH) at 500 °C. The low reactivity is due to SF 6 being kinetically inert due to:
Thermodynamically SF 6 should react with water (ΔH = -460 kJ/mol), but the rate factors are too great. Sulfur hexafluoride can be reduced with sodium in liquid ammonia, [link] , or with LiAlH 4 . In each of these reactions the mechanism involves the formation of a radical, [link] . The reaction with sulfur trioxide yields SO 2 F 2 , [link] , however, the reactions with carbon or CS 2 only occur at elevated temperatures (500 °C) and pressure (4000 atm).
Although the hexachloride is unknown, it is possible to isolate the monochloride derivative (SF 5 Cl) by the oxidative addition of Cl-F across SF 4 .
Sulfur monochloride pentafluoride is a gas (Bp = -21 °C), but unlike SF 6 it is fairly reactive due to the polarization of the S-Cl bond ( [link] ), and as a consequence it reacts with water, [link] .
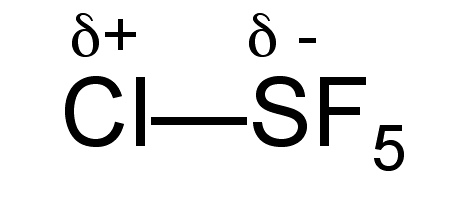
Although SF 5 does not exist as a stable molecule, the gaseous dimmer S 2 F 10 (Bp = 29 °C) may be isolated from the photochemical hydrogen reduction of SF 5 Cl, [link] .
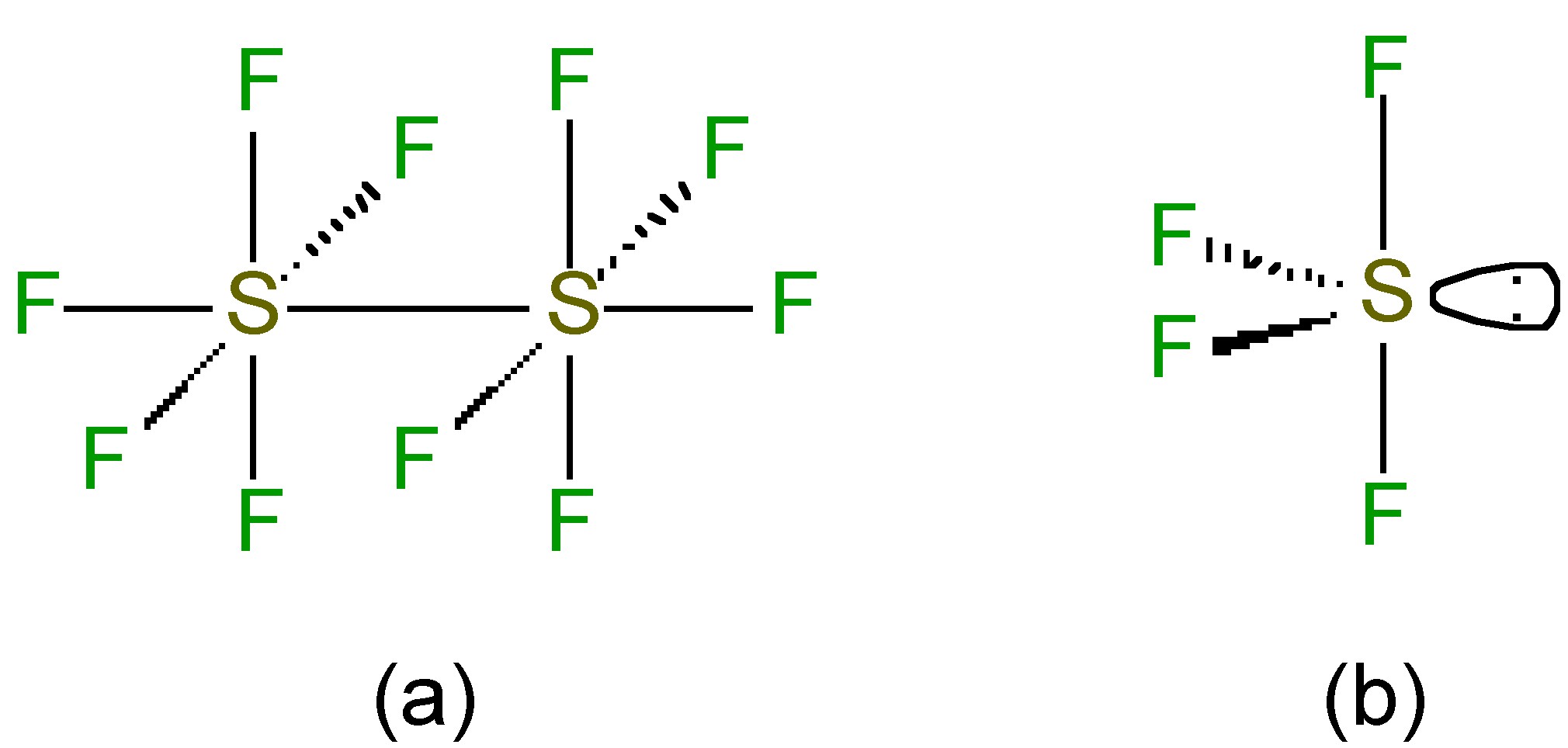
While the sulfur is octahedral in S 2 F 10 ( [link] a) the S-S bond is weak and long (2.21 Å versus an expected 2.08 Å for a single S-S bond). Despite the apparently weak S-S bond, S 2 F 10 shows almost no reactivity at room temperature; however, the S-S bond undergoes homoleptic cleavage at high temperatures. The resultant SF 5 . radicals disproportionate to give highly reactive fluoride radicals, [link] , which is the source of the highly oxidative properties of S 2 F 10 .
The SF 5 . fragment is stabilized by the addition of an alkyl radical, and thus, there are a large number of RSF 5 derivatives known. Unlike, the chloride analog, these are very stable.
Sulfur tetrafluoride (SF 4 ) is prepared from sulfur dichloride and sodium fluoride in acetonitrile solution at 70 - 80 °C.
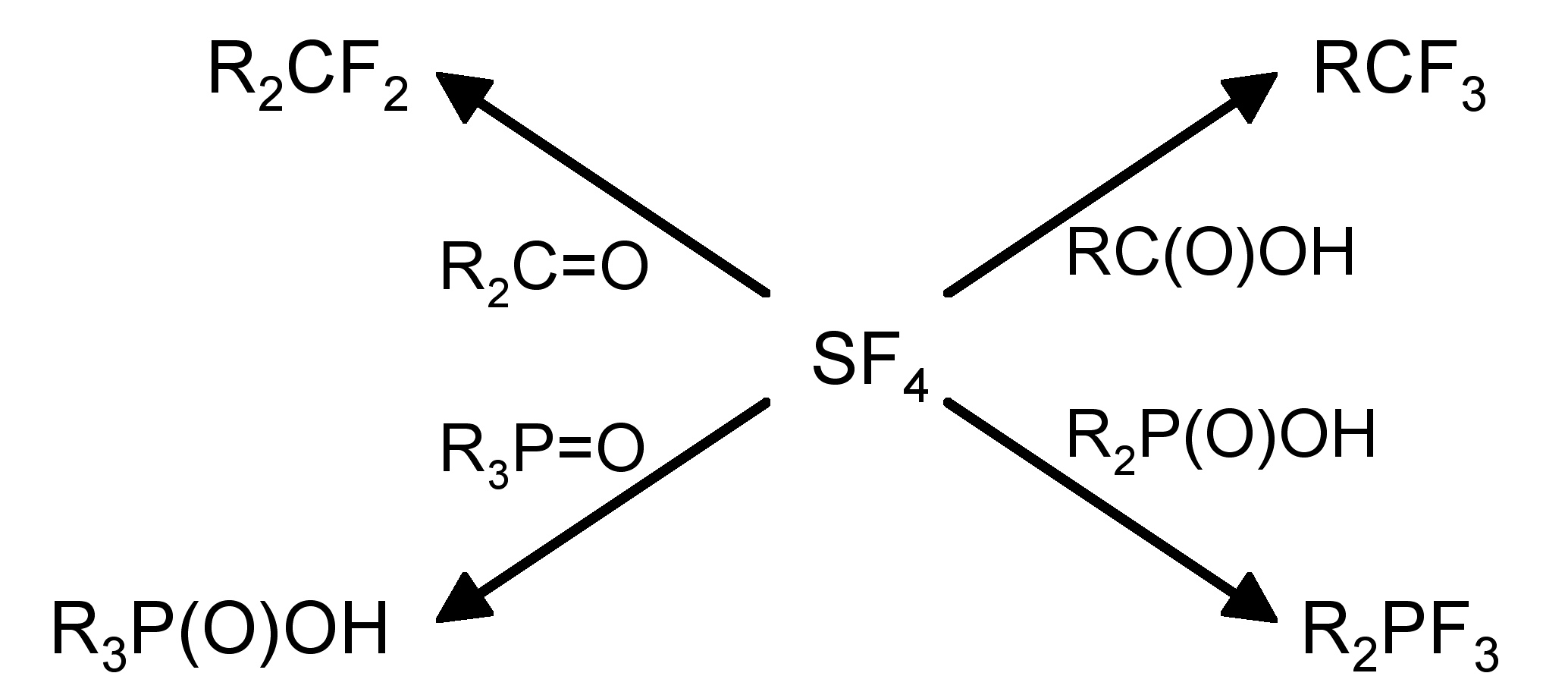
The structure of SF 4 (and its substituted derivatives RSF 3 ) is based upon a trigonal bipyramidal structure with one of the equatorial sites being occupied by a lone pair ( [link] b). Unlike the hexafluoride, sulfur tetrachloride is a highly reactive compound. It hydrolyzes readily, [link] , and is a useful fluorinating agent ( [link] ).
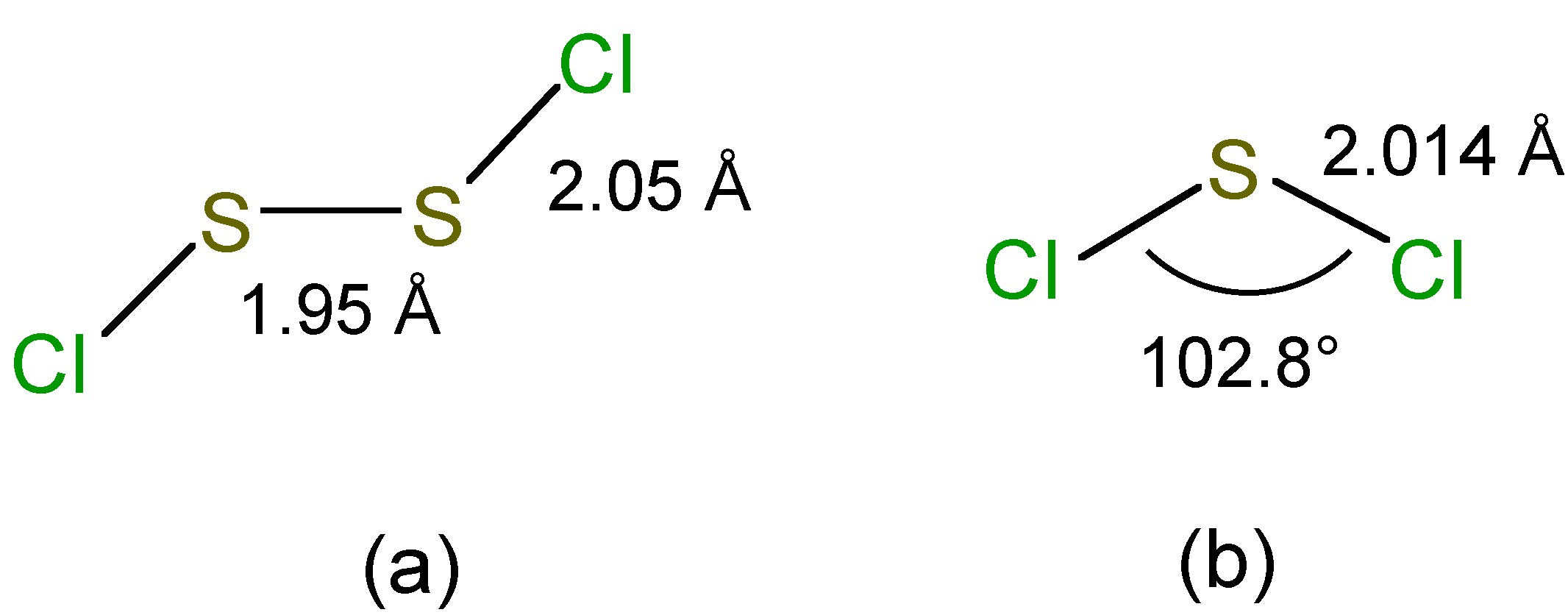
The chlorination of molten sulfur yields the fowl smelling disulfur dichloride (S 2 Cl 2 ). If the reaction is carried out with a catalyst such as FeCl 3 , SnI 4 or I 2 , an equilibrium mixture containing sulfur dichloride (SCl 2 ) is formed. However, the dichloride dissociates readily, [link] , although it can be isolated as a dark red liquid if it distilled in the presence of PCl 5 . The reaction of chlorine at -80 °C with SCl 2 or S 2 Cl 2 allows for the formation of SCl 4 as a yellow crystalline compound which dissociates above -31 °C. Sulfur chlorides are readily hydrolyzed. Sulfur chlorides are used to dissolve sulfur (giving species up to S 100 Cl 2 ) for the vulcanization of rubber.
In the vapor phase S 2 Cl 2 has C 2 symmetry ( [link] a) while that of SCl 2 has C 2v symmetry ( [link] b).

Notification Switch
Would you like to follow the 'Chemistry of the main group elements' conversation and receive update notifications?