| << Chapter < Page | Chapter >> Page > |
The combustion of sulfur results in the formation of gaseous sulfur dioxide, [link] .
The bent structure of SO 2 is shown in [link] , and as a consequence of the sp 2 hybridization the molecule is polar.
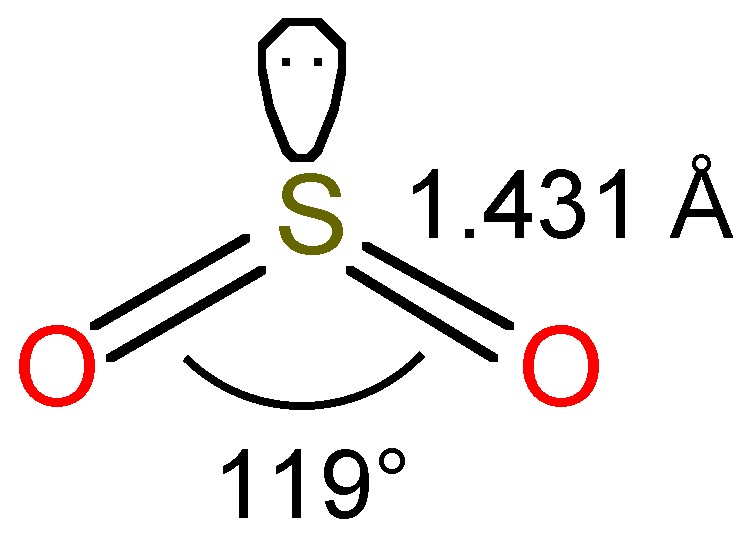
The modest boiling temperature of SO 2 (-10 °C) means that it is readily liquefied and easily kept as a liquid at room temperature under a slight pressure. The liquid is associated by dipole-dipole attractions due to the polar nature of SO 2 . Liquid SO 2 is a good solvent due to the polarity of the molecule; as a consequence it readily solubalizes polar compounds and salts. It is also convenient since it is easy to remove from reaction products by evaporation.
Sulfur dioxide is soluble in water forming aqueous solutions where most of the SO 2 is maintained as a hydrogen-bonded hydrate, in a similar manner to that observed for aqueous solutions of carbon dioxide. At equilibrium in neutral water (no added base) a small fraction reacts, to give a mixture of bisulfite (HSO 3 - , [link] a) and sulfite (SO 3 2- , [link] b), [link] . The free acid does not to exist.
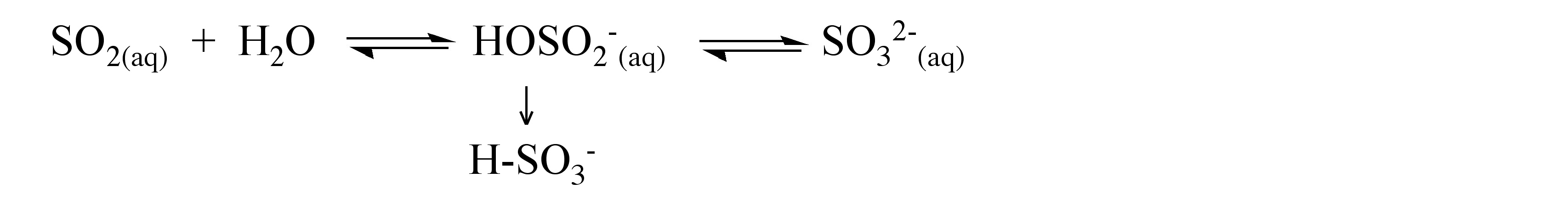
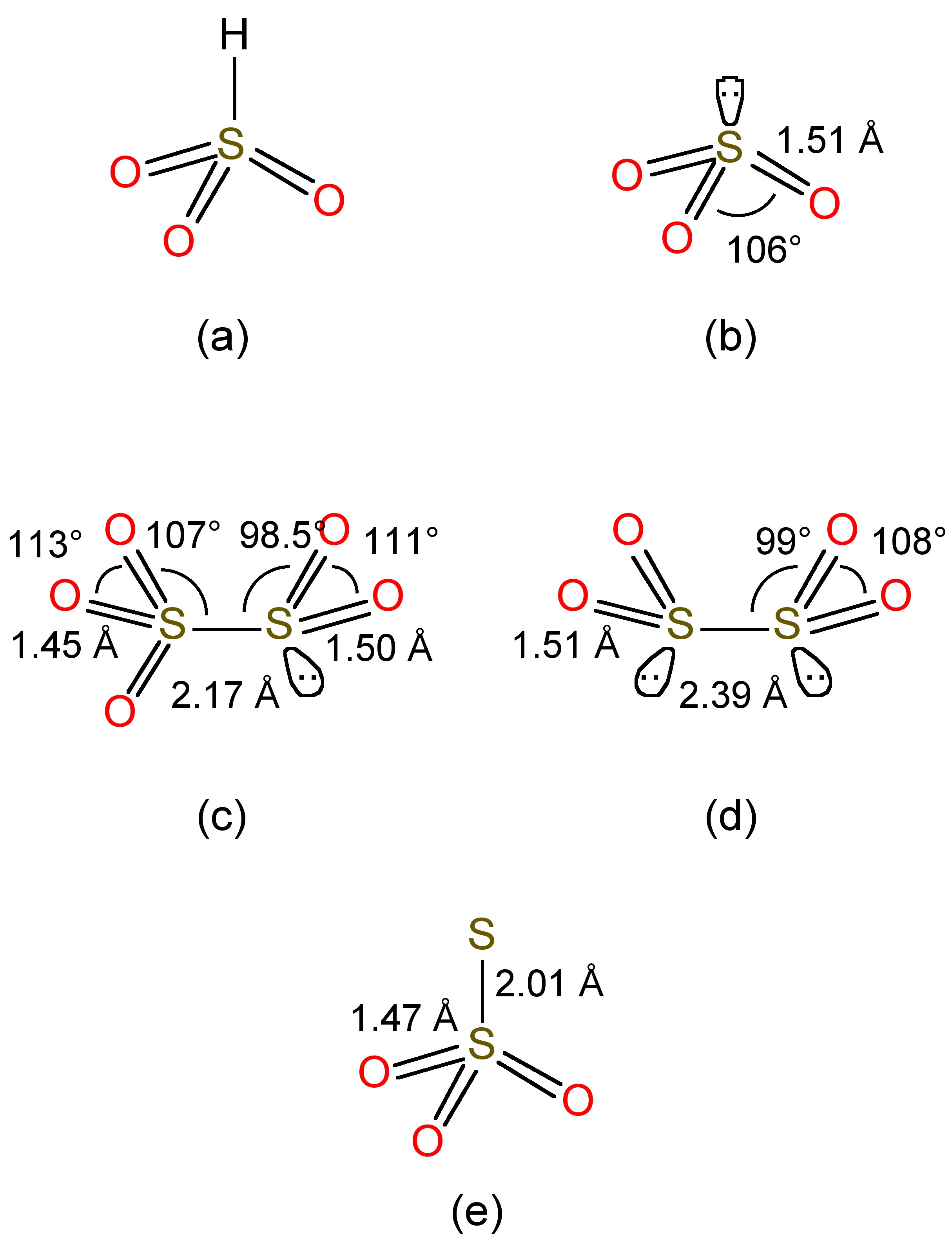
Bisulfite undergoes a further equilibrium, [link] , to form disulfite, whose structure is shown in [link] c.
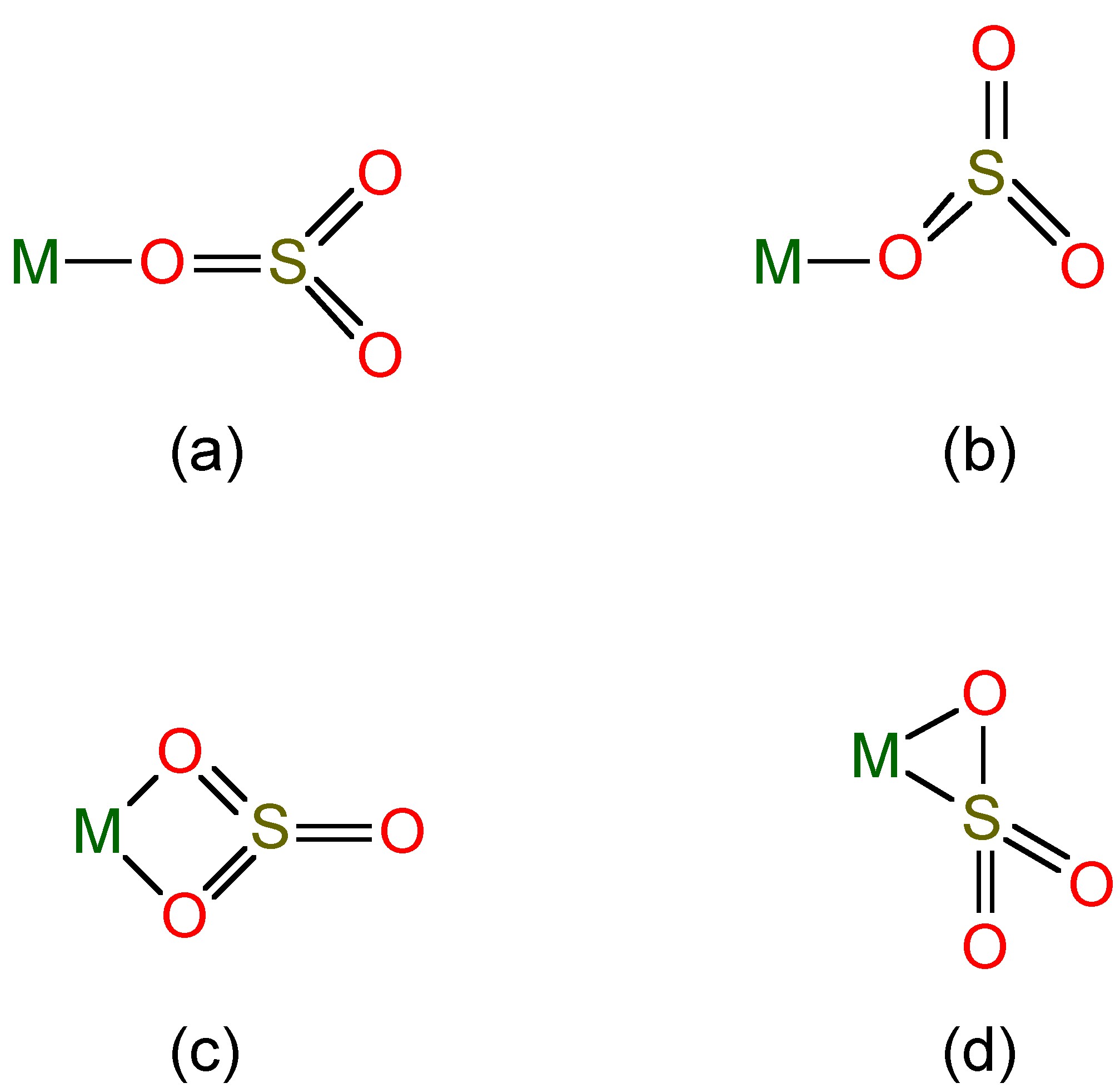
Salts of these anions are known, and complexes of the sulfite ion are known ( [link] ), while SO 2 itself can act as a ligand to heavy metals.
The bisulfite ion has strong reducing properties, e.g., [link] and [link] .
Bisulfite is also reduced by zinc in the presence of additional SO 2 , [link] , to form the highly reducing dithionite anion ( [link] d). Reaction of bisulfite with elemental sulfur yields the thiosulfate anion ( [link] e), [link] .
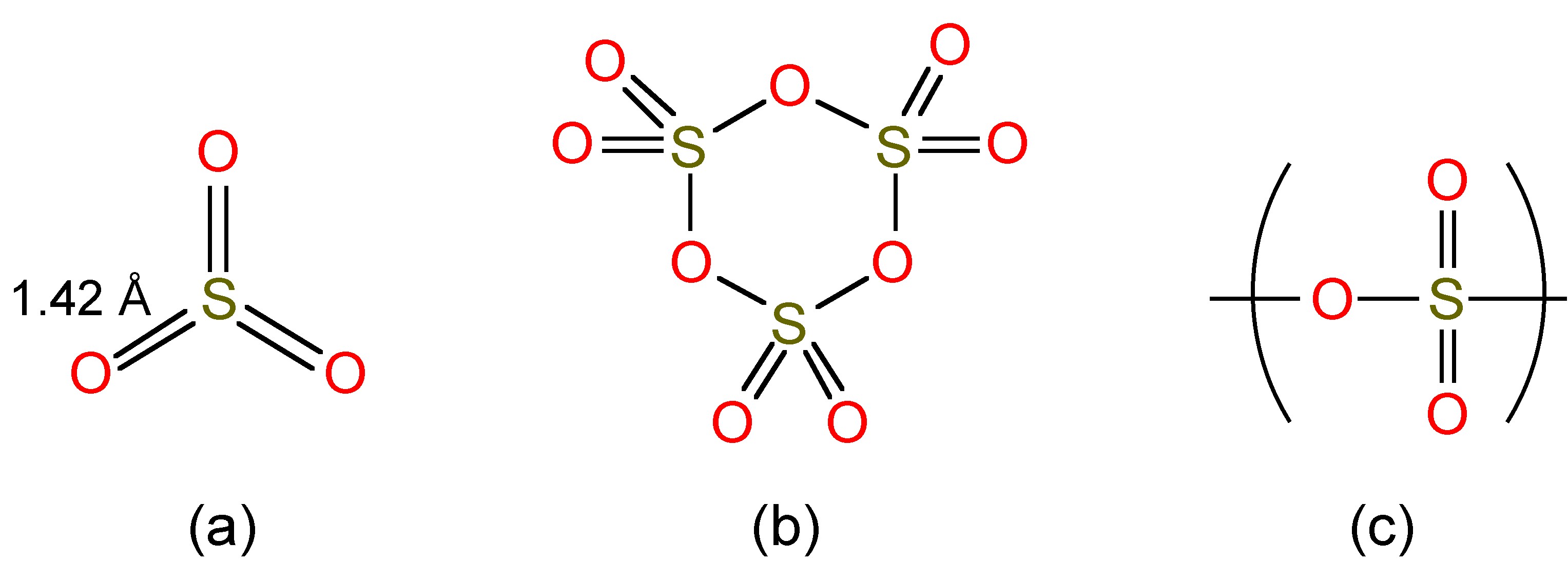
Oxidation of sulfur dioxide in the presence of a catalyst (e.g., platinum) yields sulfur trioxide, [link] , which may be condensed to a liquid at room temperature (Bp = 45 °C).
Liquid SO 3 exists as a mixture of monomer and trimers ( [link] a and b), while as a solid (Mp = 16.9 °C) it forms polymers ( [link] c).
The reaction of SO 3 with water results in the formation of sulfuric acid, H 2 SO 4 , as a viscous, hydrogen bonded liquid. Sulfuric acid is a strong protic acid, which in dilute solutions (in water) reacts as a dibasic acid, [link] , forming bisulfate (HSO 4 - ) and sulfate (SO 4 2- ) anions. A large number of salts are known for both anions. In addition, sulfate is known to act as a monodentate or bidentate ligand in coordination complexes.
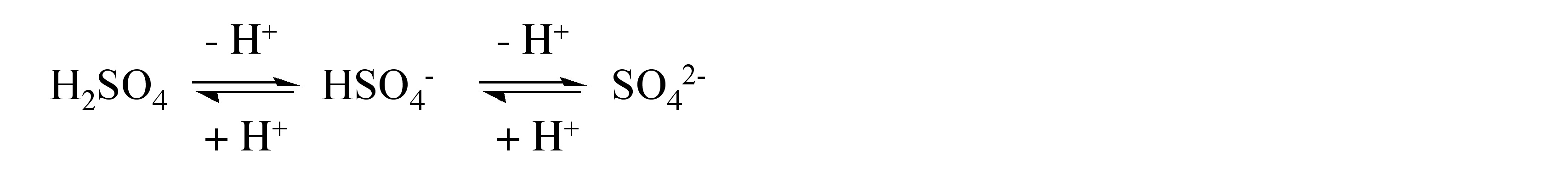
The dissolution of SO 3 in concentrated sulfuric acid yields very corrosive, fuming sulfuric acid, which contains some pyrosulfuric acid, [link] .
Warning: The corrosive properties of sulfuric acid are accentuated by its highly exothermic reaction with water. Burns from sulfuric acid are potentially more serious than those of comparable strong acids (e.g., hydrochloric acid), as there is additional tissue damage due to dehydration and particularly secondary thermal damage due to the heat liberated by the reaction with water.
Sulfur dioxide is formed as a pollutant during the combustion of sulfur containing fuels, in particular coal. While the emission of SO 2 itself leads to concerns it is its conversion to sulfuric acid in the form of acid rain that has been of concern for several decades. The pathway for the formation of sulfuric acid in the atmosphere is depandant on whether the reaction occurs in dry atmosphere or in clouds and rain.
In the dry atmosphere, gaseous sulfur dioxide reacts with the hydroxide radical (formed by the photochemical decomposition of ozone, [link] and [link] ) in the presence of a non-reactive gas molecule such as nitrogen, [link] . The sulfurous acid, thus formed reacts with oxygen to generate sulfur trioxide, [link] , which reacts with water to form sulfuric acid, [link] .
Measurements indicate that the conversion rate of SO 2 to H 2 SO 4 is 4% per hour on a clear sunny day, but the rate is slower during the winter.
In the liquid phase SO 2 reacts directly with water, [link] . The bisulfite (HSO 3 - ) is oxidized by hydrogen peroxide forming a forming bisulfate (HSO 4 - ) solution, [link] .
Water soluble hydrogen peroxide is formed by the oxidation of water, [link] .
The HO 2 radical is formed by the photolysis of organic carbonyl compounds, e.g., formaldehyde in [link] and [link] .
The conversion rate is independent of pH is very fast: almost 100% per hour in summer. However, the conversion is limited by the supply of hydrogen peroxide, which is often present in much lower levels than SO 2 . Thus, a reduction in sulfur dioxide emissions does not always correlate with a reduction of wet acid deposition.

Notification Switch
Would you like to follow the 'Chemistry of the main group elements' conversation and receive update notifications?