| << Chapter < Page | Chapter >> Page > |
There are many functional groups found in chemistry, each of which gives specific properties to the class of molecules containing it.
| Functional Group | Functional Group Structure | Functional Group | Functional Group Structure |
|---|---|---|---|
| Alkane |
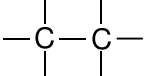 |
Amine |
 |
| Alkene |
 |
Aldehyde |
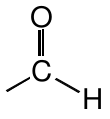 |
| Alkyne |
|
Ketone |
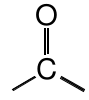 |
| Arene |
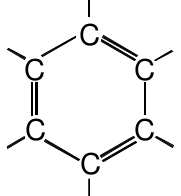 |
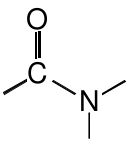
Amide |
 |
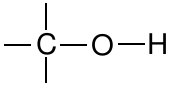
| Alcohol |
 |
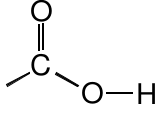
Carboxylic Acid |
 |
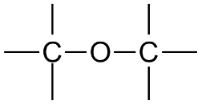
| Ether |
 |
Ester |
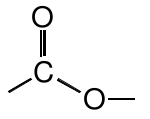 |
As mentioned above, the field of Organic Chemistry is in large part about finding ways to synthesize new molecules containing specific functional groups which therefore form compounds with specific properties we might want. Much of Biochemistry can be understood by looking at the functional groups present in biomolecules.
Earlier in this concept study, we learned that different molecules can be formed from the same set of atoms. Isomers are molecules with the same molecular formula but different molecular structures, and are therefore distinctly different compounds. Rearranging the atoms in the molecule creates a new compound with new physical and chemical properties.
What if, instead of rearranging the atoms in the molecule, we rearrange the electrons? This would possibly change where double or triple bonds are located and whether there are lone pairs of electrons or not. Does this also give rise to new compounds and therefore new isomers?
We first look at benzene, a compound with the molecular formula C 6 H 6 . For six carbon atoms, there are not very many hydrogen atoms. Compare benzene to hexane, which has the molecular formula C 6 H 14 . This means that there must be several double or triple carbon-carbon bonds in benzene. Experiments reveal to us two facts about the molecular structure of benzene. First, the six carbon atoms are arranged in a ring, not a chain, and each carbon atom is bonded to a single hydrogen atom. Second, the bonds between the carbon atoms in the ring all have the same length as one another. The second observation tells us that, somehow, all of the bonds in benzene are identical to each other.
The correct molecular structure which explained these observations was a puzzle for chemists. A structure in which the six carbon atoms are arranged in a ring would be:
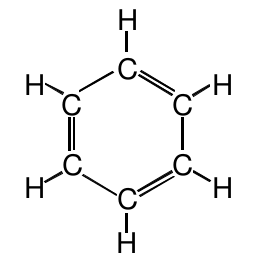
This structure cannot be the right structure though. Although it satisfies the octet rule and the valences of the carbon atoms, it does not correctly explain why the bonds are all the same length. This structure would predict three shorter bonds and three longer bonds.
A clue to the right structure is found from the value of the bond length, 139 pm. This length is between the typical length of a single bond, 153 pm, and the typical length of a double bond, 134 pm. This is a confusing clue: it suggests that the bonds in benzene are neither single bonds nor double bonds. We clearly need to expand on our model of Lewis structures.
If we look at the molecular structure proposed above, we can see that we made an arbitrary choice of where to put the double bonds. To satisfy the valences of the carbon atoms, we need to alternate the double bonds and single bonds, but we could have chosen the other three C-C bonds to be double:

Notification Switch
Would you like to follow the 'Concept development studies in chemistry 2012' conversation and receive update notifications?