| << Chapter < Page | Chapter >> Page > |
Elements
http://smallfry.dmu.ac.uk/chem/tables
Listen attentively to the educator’s explanation and complete the following.
The Periodic Table
1. The horizontal lines are known as
2. The vertical lines are known as
3. In what part of the periodic table do we find the metals?
4. Which metals are stored under paraffin? Why?
5. What do we call these metals?
6. In what part of the periodic table do we find the non-metals?
7. Which non-metal is stored under water? Why?
8. Where do we find most of the gases? Explain this
9. Which gases do not react with other substances?
10. What is the name of this group of gases?
11. Name the lightest gas
12. Which elements occur in liquid phase at room temperature?
13. Which element sublimates?
14. In which phase do most elements occur at room temperature?
15. Why do we place particular elements in the same group?
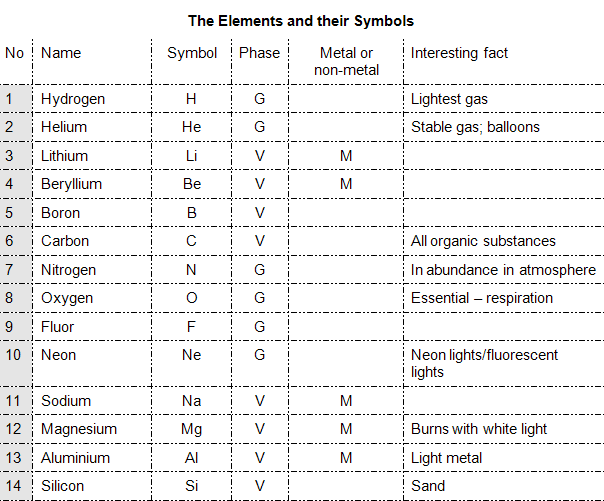
Elements and their Symbols
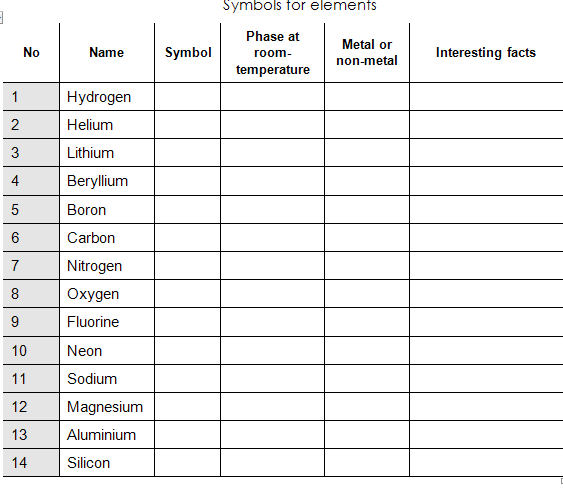
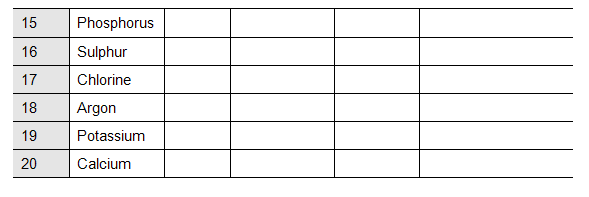
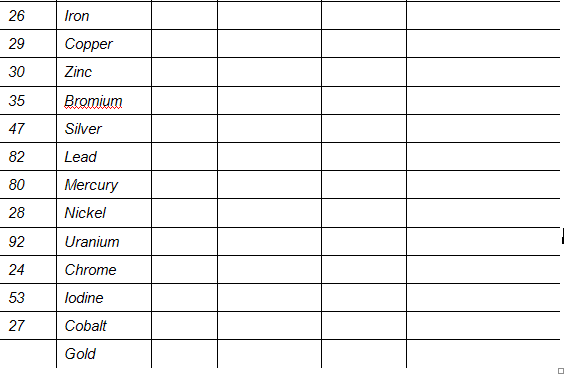
Assessment of listening skills
Test yourself: are you able to answer questions 1 – 15 without referring to the answers?
[LO 2.1]
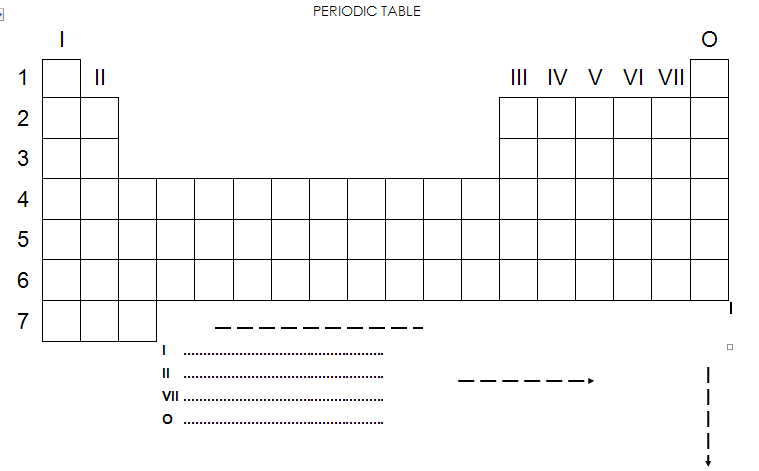
Any question that cannot be answered must be investigated.
Which element do we have here ?
[LO 2.1]
Do you know the following?
Some elements, like
CARBON
occur in various forms:
diamonds
coal, and the
graphite in your pencil!
http://education.jlab.org/itselemental/ele006
http://mineral.galleries.com
Learning outcome 2: Constructing science knowledge
The learner will know and be able to interpret and apply scientific, technological and environmental knowledge.
We know this when the learner
2.1 is able to recall significant information;
2.2 is able to categorise information.
1. periods
2. groups
3. below left and in the transition block
4. Li, Na, K – reactive with water vapour
5. Alkali metals
6. Below right
7. P – reactive with oxygen
8. On the extreme right
9. Gases on extreme right
10. Noble gases
11. H
12. Br and Hg
13. Iodine
14. Solid substance
15. Corresponding qualities
The PERIODIC TABLE OF ELEMENTS
1. periods
2. groups
3. below left and in the transition block
4. Li, Na, K – reactive with water vapour
5. Alkali metals
6. Below right
7. P – reactive with oxygen
8. On the extreme right
9. Gases on extreme right
10. Noble gases
11. H
12. Br and Hg
13. Iodine
14. Solid substance
15. Corresponding qualities
ELEMENTS AS SYMBOLS
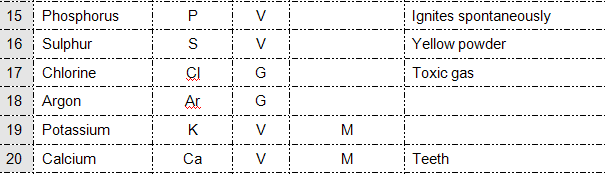
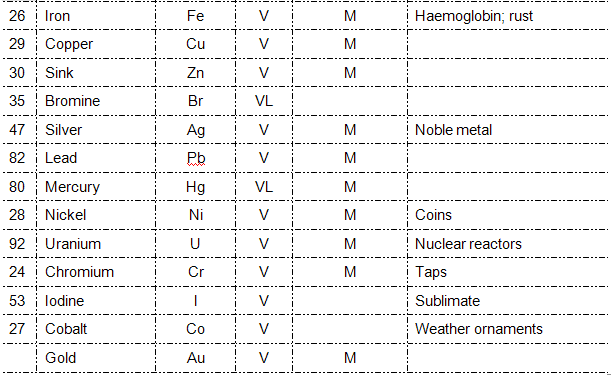
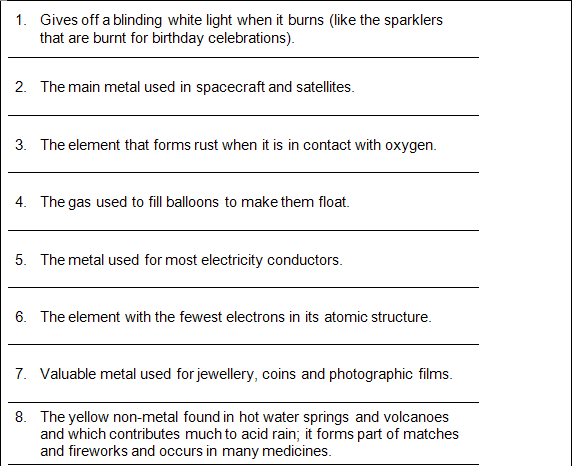
GROUP WORK:QUIZ
1. Mg
2. Titanium
3. Fe
4. He
5. Cu
6. H
7. Ag
8. S
9. Br
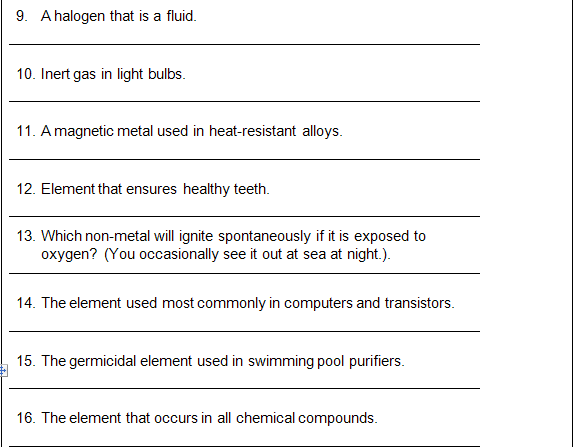
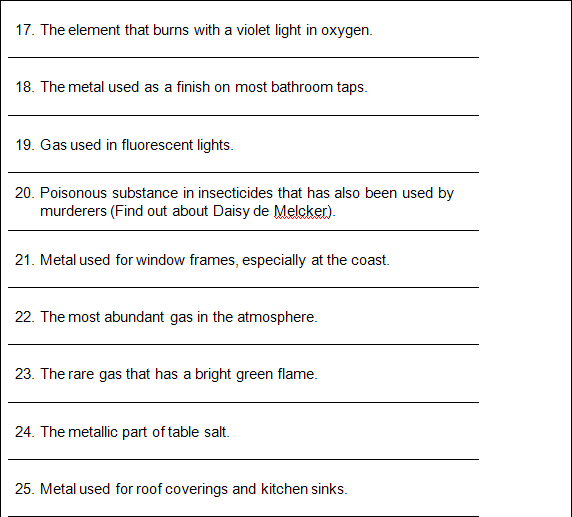
10. Ar
11. Co
12. Ca
13. P
14. Si
16. Cl
17. K
18. Cr
19. Ne
20. Cyanide
21. Al
22. N
23. Kr
24. Na
25. Zinc
CLASS ACTIVITY: RECOGNITION OF METALS AND NON-METALS
Metals:
Non-metals:
1. shiny, hard
2. gold
3. demand
4. study of metals
5. alloy
6.
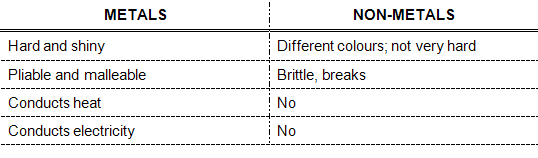
7. Pliable – long threads stretched out.
Malleable – hammered into thin plates/sheets.
GROUP WORK: QUIZ
1. Mg
2. Titanium
3. Fe
4. He
5. Cu
6. H
7. Ag
8. S
9. Br
10. Ar
11. Co
12. Ca
13. P
14. Si
16. Cl
17. K
18. Cr
19. Ne
20. Cyanide
21. Al
22. N
23. Kr
24. Na
25. Zinc
| GoldAuVM |

Notification Switch
Would you like to follow the 'Natural sciences grade 8' conversation and receive update notifications?