| << Chapter < Page | Chapter >> Page > |
In a Lewis structure, formal charges can be assigned to each atom by treating each bond as if one-half of the electrons are assigned to each atom. These hypothetical formal charges are a guide to determining the most appropriate Lewis structure. A structure in which the formal charges are as close to zero as possible is preferred. Resonance occurs in cases where two or more Lewis structures with identical arrangements of atoms but different distributions of electrons can be written. The actual distribution of electrons (the resonance hybrid) is an average of the distribution indicated by the individual Lewis structures (the resonance forms).
Write resonance forms that describe the distribution of electrons in each of these molecules or ions.
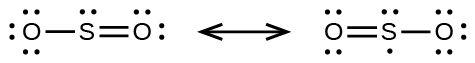
(a) selenium dioxide, OSeO
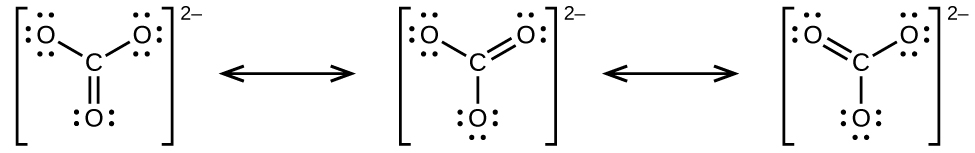
(b) nitrate ion,
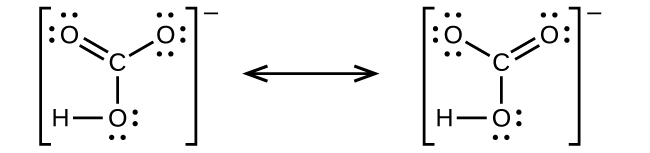
(c) nitric acid, HNO 3 (N is bonded to an OH group and two O atoms)
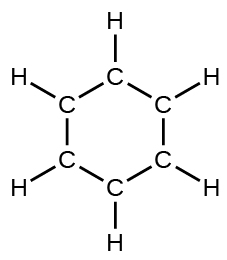
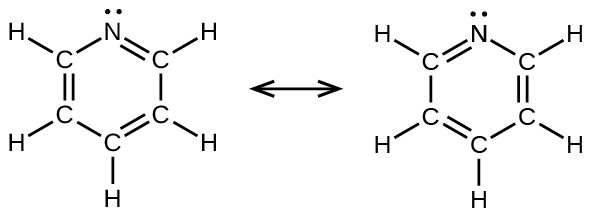
(d) benzene, C 6 H 6 :
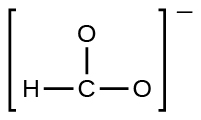
(e) the formate ion:
Write resonance forms that describe the distribution of electrons in each of these molecules or ions.
(a) sulfur dioxide, SO 2
(b) carbonate ion,
(c) hydrogen carbonate ion, (C is bonded to an OH group and two O atoms)
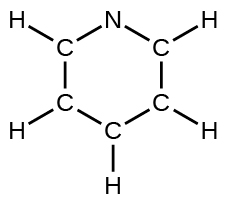
(d) pyridine:
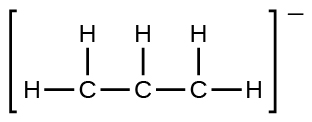
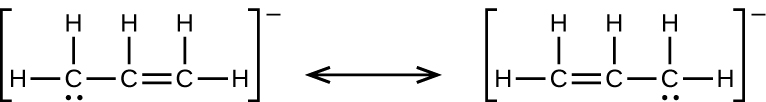
(e) the allyl ion:
(a)
 ;
;
(b)
 ;
;
(c)
 ;
;
(d)
 ;
;
(e)
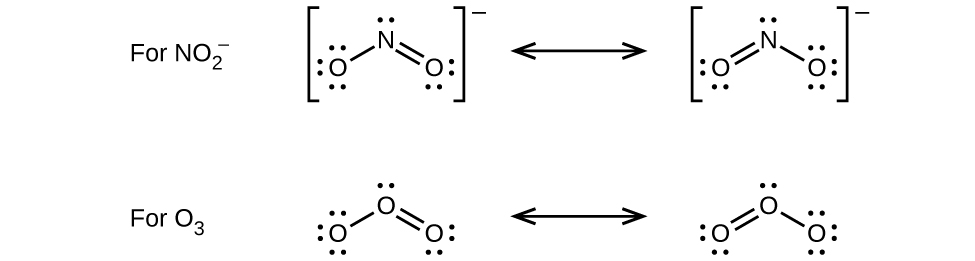
Write the resonance forms of ozone, O 3 , the component of the upper atmosphere that protects the Earth from ultraviolet radiation.
Sodium nitrite, which has been used to preserve bacon and other meats, is an ionic compound. Write the resonance forms of the nitrite ion,
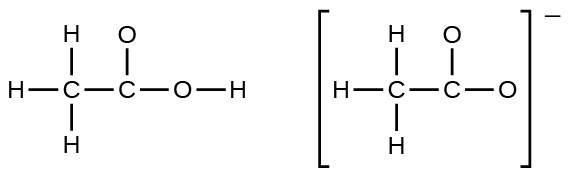
In terms of the bonds present, explain why acetic acid, CH 3 CO 2 H, contains two distinct types of carbon-oxygen bonds, whereas the acetate ion, formed by loss of a hydrogen ion from acetic acid, only contains one type of carbon-oxygen bond. The skeleton structures of these species are shown:
Write the Lewis structures for the following, and include resonance structures where appropriate. Indicate which has the strongest carbon-oxygen bond.
(a) CO 2
(b) CO
(a)
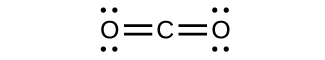
(b)
![]()
CO has the strongest carbon-oxygen bond because there is a triple bond joining C and O. CO
2 has double bonds.
Toothpastes containing sodium hydrogen carbonate (sodium bicarbonate) and hydrogen peroxide are widely used. Write Lewis structures for the hydrogen carbonate ion and hydrogen peroxide molecule, with resonance forms where appropriate.
Determine the formal charge of each element in the following:
(a) HCl
(b) CF 4
(c) PCl 3
(d) PF 5
(a) H: 0, Cl: 0; (b) C: 0, F: 0; (c) P: 0, Cl 0; (d) P: 0, F: 0
Determine the formal charge of each element in the following:
(a) H 3 O +
(b)
(c) NH 3
(d)
(e) H 2 O 2
Calculate the formal charge of chlorine in the molecules Cl 2 , BeCl 2 , and ClF 5 .
Cl in Cl 2 : 0; Cl in BeCl 2 : 0; Cl in ClF 5 : 0
Calculate the formal charge of each element in the following compounds and ions:
(a) F 2 CO
(b) NO –
(c)
(d)
(e) H 2 CCH 2
(f) ClF 3
(g) SeF 6
(h)
Draw all possible resonance structures for each of these compounds. Determine the formal charge on each atom in each of the resonance structures:
(a) O 3
(b) SO 2
(c)
(d)
(a)
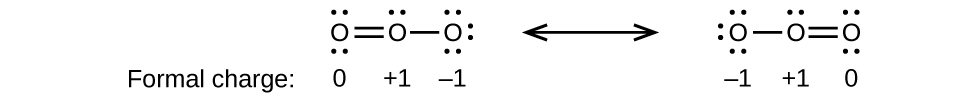 ;
;
(b)
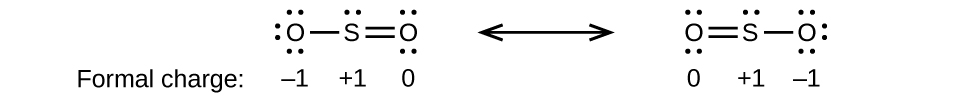 ;
;
(c)
![[Two Lewis structures are shown, with brackets surrounding each with a superscripted negative sign and a double ended arrow in between. The left structure shows a nitrogen atom with one lone pair of electrons single bonded to an oxygen atom with three lone pairs of electrons and double bonded to an oxygen atom with two lone pairs of electrons. The symbols and numbers below this structure read “open parenthesis, 0, close parenthesis, open parenthesis, 0, close parenthesis, open parenthesis, negative 1, close parenthesis. The right structure appears as a mirror image of the left and the symbols and numbers below this structure read “open parenthesis, negative 1, close parenthesis, open parenthesis, 0, close parenthesis, open parenthesis, 0, close parenthesis.]](/ocw/mirror/col11760/m51051/CNX_Chem_07_04_Exercis12c_img.jpg) ;
;
(d)
![[Three Lewis structures are shown, with brackets surrounding each with a superscripted negative sign and a double ended arrow in between. The left structure shows a nitrogen atom single bonded to two oxygen atoms, each with three lone pairs of electrons and double bonded to an oxygen atom with two lone pairs of electrons. The single bonded oxygen atoms are labeled, from the top of the structure and going clockwise, “open parenthesis, negative 1, close parenthesis, open parenthesis, positive 1, close parenthesis”. The symbols and numbers below this structure read “open parenthesis, 0, close parenthesis, open parenthesis, negative 1, close parenthesis. The middle structure shows a nitrogen atom single bonded to two oxygen atoms, each with three lone pairs of electrons, one of which is labeled “open parenthesis, positive 1, close parenthesis” and double bonded to an oxygen atom with two lone pairs of electrons labeled “open parenthesis, 0, close parenthesis”. The symbols and numbers below this structure read “open parenthesis, negative 1, close parenthesis, open parenthesis, negative 1, close parenthesis. The right structure shows a nitrogen atom single bonded to two oxygen atoms, each with three lone pairs of electrons and double bonded to an oxygen atom with two lone pairs of electrons. One of the single bonded oxygen atoms is labeled, “open parenthesis, negative 1, close parenthesis while the double bonded oxygen is labeled, “open parenthesis, positive 1, close parenthesis”. The symbols and numbers below this structure read “open parenthesis, negative 1, close parenthesis” and “open parenthesis, 0, close parenthesis”.]](/ocw/mirror/col11760/m51051/CNX_Chem_07_04_Exercis12d_img.jpg)
Based on formal charge considerations, which of the following would likely be the correct arrangement of atoms in nitrosyl chloride: ClNO or ClON?
Based on formal charge considerations, which of the following would likely be the correct arrangement of atoms in hypochlorous acid: HOCl or OClH?
HOCl
Based on formal charge considerations, which of the following would likely be the correct arrangement of atoms in sulfur dioxide: OSO or SOO?
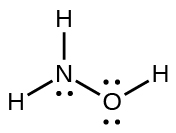
Draw the structure of hydroxylamine, H 3 NO, and assign formal charges; look up the structure. Is the actual structure consistent with the formal charges?
The structure that gives zero formal charges is consistent with the actual structure:
Iodine forms a series of fluorides (listed here). Write Lewis structures for each of the four compounds and determine the formal charge of the iodine atom in each molecule:
(a) IF
(b) IF 3
(c) IF 5
(d) IF 7
Write the Lewis structure and chemical formula of the compound with a molar mass of about 70 g/mol that contains 19.7% nitrogen and 80.3% fluorine by mass, and determine the formal charge of the atoms in this compound.
NF
3 ;
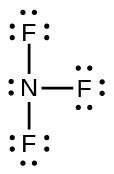
Which of the following structures would we expect for nitrous acid? Determine the formal charges:
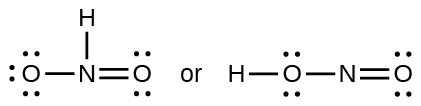
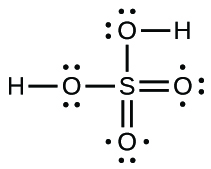
Sulfuric acid is the industrial chemical produced in greatest quantity worldwide. About 90 billion pounds are produced each year in the United States alone. Write the Lewis structure for sulfuric acid, H 2 SO 4 , which has two oxygen atoms and two OH groups bonded to the sulfur.

Notification Switch
Would you like to follow the 'Chemistry' conversation and receive update notifications?