| << Chapter < Page | Chapter >> Page > |
The benefit of the bicarbonate buffer system is that carbon dioxide is “soaked up” into the blood with little change to the pH of the system. This is important because it takes only a small change in the overall pH of the body for severe injury or death to result. The presence of this bicarbonate buffer system also allows for people to travel and live at high altitudes: When the partial pressure of oxygen and carbon dioxide change at high altitudes, the bicarbonate buffer system adjusts to regulate carbon dioxide while maintaining the correct pH in the body.
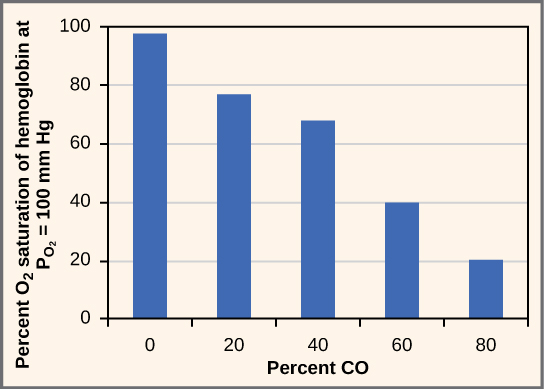
While carbon dioxide can readily associate and dissociate from hemoglobin, other molecules such as carbon monoxide (CO) cannot. Carbon monoxide has a greater affinity for hemoglobin than oxygen. Therefore, when carbon monoxide is present, it binds to hemoglobin preferentially over oxygen. As a result, oxygen cannot bind to hemoglobin, so very little oxygen is transported through the body ( [link] ). Carbon monoxide is a colorless, odorless gas and is therefore difficult to detect. It is produced by gas-powered vehicles and tools. Carbon monoxide can cause headaches, confusion, and nausea; long-term exposure can cause brain damage or death. Administering 100 percent (pure) oxygen is the usual treatment for carbon monoxide poisoning. Administration of pure oxygen speeds up the separation of carbon monoxide from hemoglobin.
Hemoglobin is a protein found in red blood cells that is comprised of two alpha and two beta subunits that surround an iron-containing heme group. Oxygen readily binds this heme group. The ability of oxygen to bind increases as more oxygen molecules are bound to heme. Disease states and altered conditions in the body can affect the binding ability of oxygen, and increase or decrease its ability to dissociate from hemoglobin.
Carbon dioxide can be transported through the blood via three methods. It is dissolved directly in the blood, bound to plasma proteins or hemoglobin, or converted into bicarbonate. The majority of carbon dioxide is transported as part of the bicarbonate system. Carbon dioxide diffuses into red blood cells. Inside, carbonic anhydrase converts carbon dioxide into carbonic acid (H 2 CO 3 ), which is subsequently hydrolyzed into bicarbonate and H + . The H + ion binds to hemoglobin in red blood cells, and bicarbonate is transported out of the red blood cells in exchange for a chloride ion. This is called the chloride shift. Bicarbonate leaves the red blood cells and enters the blood plasma. In the lungs, bicarbonate is transported back into the red blood cells in exchange for chloride. The H + dissociates from hemoglobin and combines with bicarbonate to form carbonic acid with the help of carbonic anhydrase, which further catalyzes the reaction to convert carbonic acid back into carbon dioxide and water. The carbon dioxide is then expelled from the lungs.
[link] The kidneys are responsible for removing excess H+ ions from the blood. If the kidneys fail, what would happen to blood pH and to hemoglobin affinity for oxygen?
[link] The blood pH will drop and hemoglobin affinity for oxygen will decrease.

Notification Switch
Would you like to follow the 'Biology' conversation and receive update notifications?