| << Chapter < Page | Chapter >> Page > |
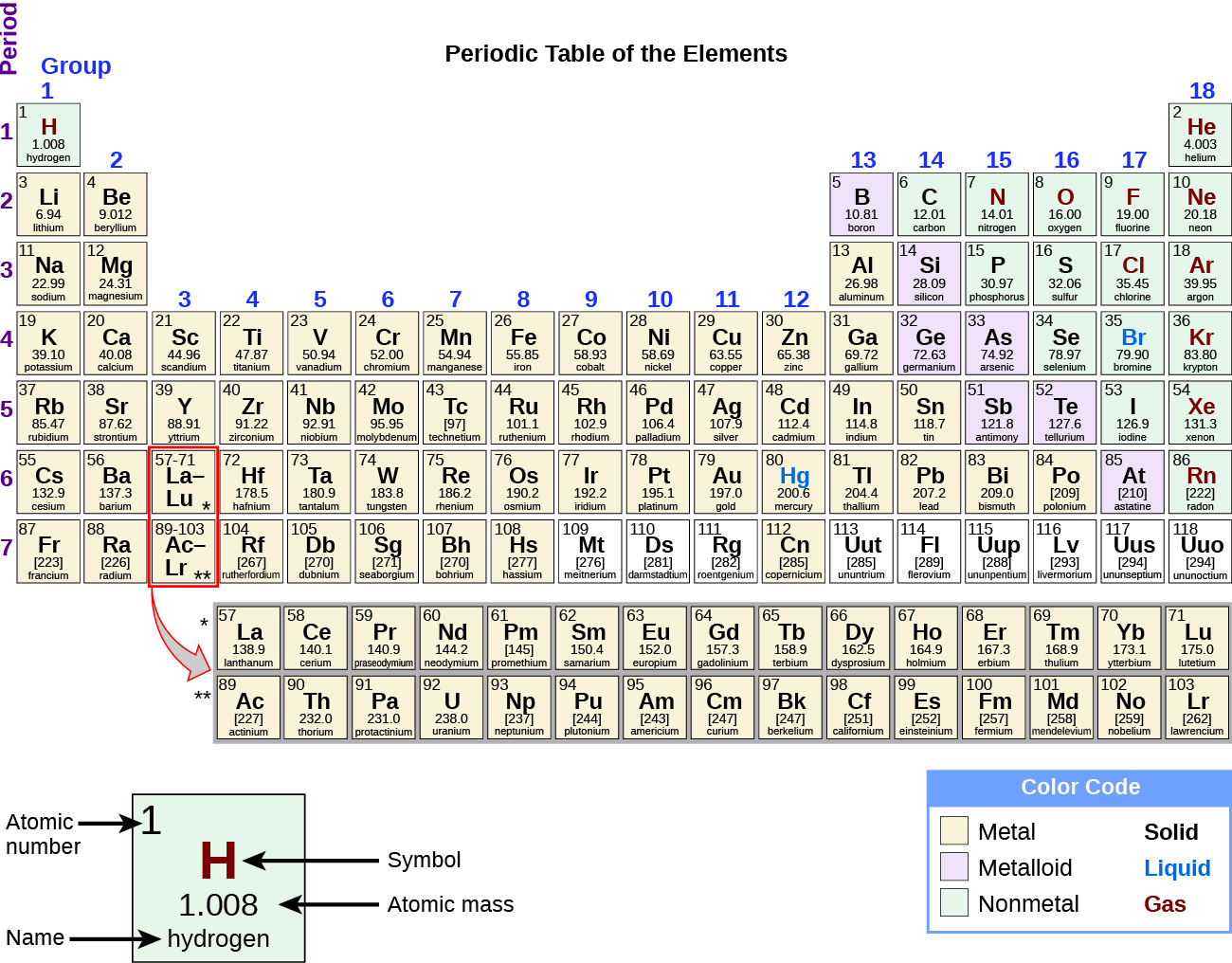
Many elements differ dramatically in their chemical and physical properties, but some elements are similar in their behaviors. For example, many elements appear shiny, are malleable (able to be deformed without breaking) and ductile (can be drawn into wires), and conduct heat and electricity well. Other elements are not shiny, malleable, or ductile, and are poor conductors of heat and electricity. We can sort the elements into large classes with common properties: metals (elements that are shiny, malleable, good conductors of heat and electricity—shaded yellow); nonmetals (elements that appear dull, poor conductors of heat and electricity—shaded green); and metalloids (elements that conduct heat and electricity moderately well, and possess some properties of metals and some properties of nonmetals—shaded purple).
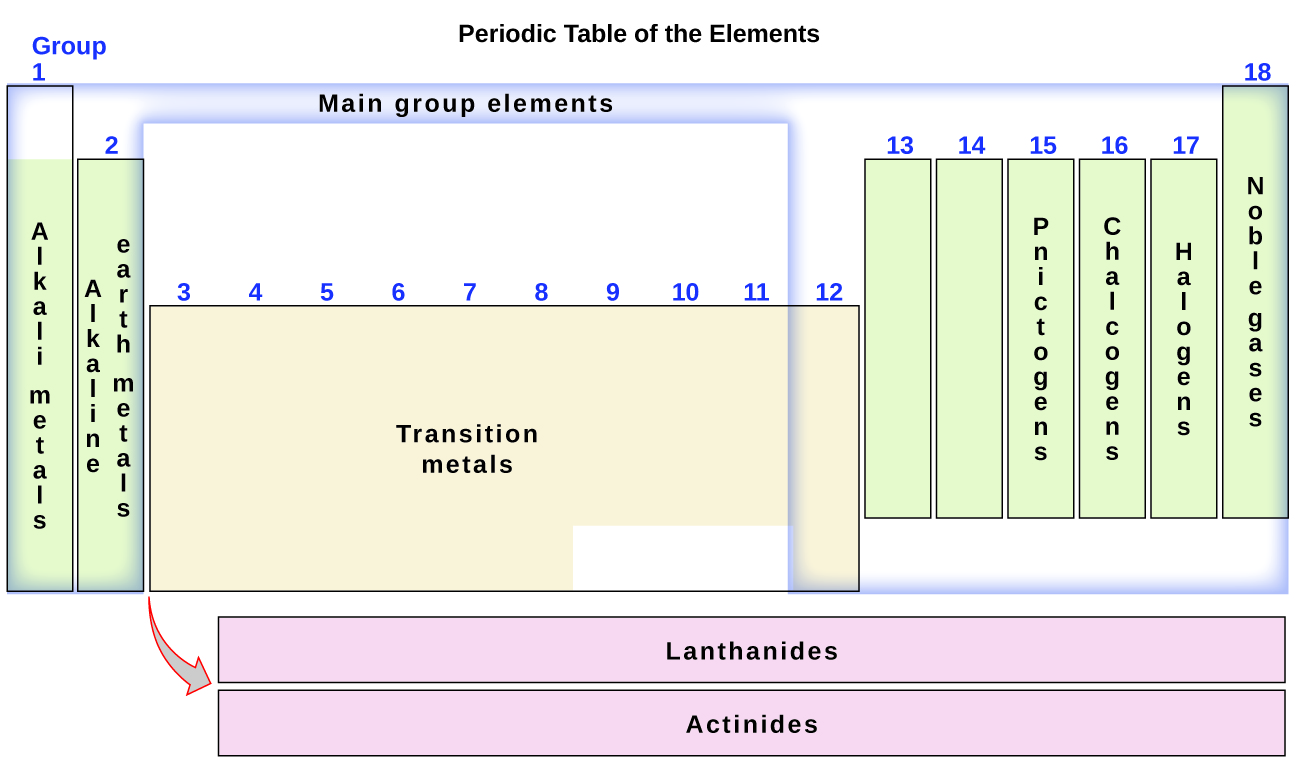
The elements can also be classified into the main-group elements (or representative elements ) in the columns labeled 1, 2, and 13–18; the transition metals in the columns labeled 3–12; and inner transition metals in the two rows at the bottom of the table (the top-row elements are called lanthanides and the bottom-row elements are actinides ; [link] ). The elements can be subdivided further by more specific properties, such as the composition of the compounds they form. For example, the elements in group 1 (the first column) form compounds that consist of one atom of the element and one atom of hydrogen. These elements (except hydrogen) are known as alkali metals , and they all have similar chemical properties. The elements in group 2 (the second column) form compounds consisting of one atom of the element and two atoms of hydrogen: These are called alkaline earth metals , with similar properties among members of that group. Other groups with specific names are the pnictogens (group 15), chalcogens (group 16), halogens (group 17), and the noble gases (group 18, also known as inert gases ). The groups can also be referred to by the first element of the group: For example, the chalcogens can be called the oxygen group or oxygen family. Hydrogen is a unique, nonmetallic element with properties similar to both group 1A and group 7A elements. For that reason, hydrogen may be shown at the top of both groups, or by itself.
Click on this link for an interactive periodic table, which you can use to explore the properties of the elements (includes podcasts and videos of each element). You may also want to try this one that shows photos of all the elements.
(a) chlorine
(b) calcium
(c) sodium
(d) sulfur
(a) halogen
(b) alkaline earth metal
(c) alkali metal
(d) chalcogen
(a) krypton
(b) selenium
(c) barium
(d) lithium
(a) noble gas; (b) chalcogen; (c) alkaline earth metal; (d) alkali metal

Notification Switch
Would you like to follow the 'Chemistry' conversation and receive update notifications?