| << Chapter < Page | Chapter >> Page > |
A molecular formula is a representation of a molecule that uses chemical symbols to indicate the types of atoms followed by subscripts to show the number of atoms of each type in the molecule. (A subscript is used only when more than one atom of a given type is present.) Molecular formulas are also used as abbreviations for the names of compounds.
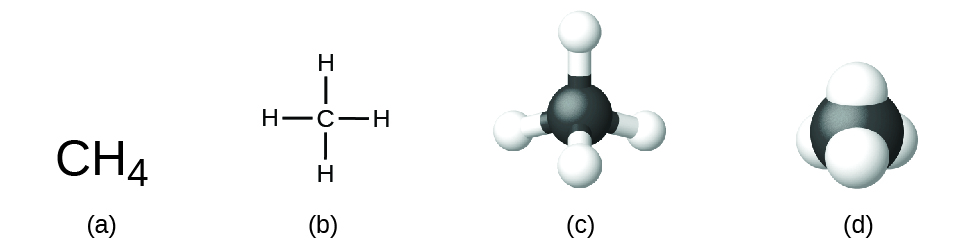
The structural formula for a compound gives the same information as its molecular formula (the types and numbers of atoms in the molecule) but also shows how the atoms are connected in the molecule. The structural formula for methane contains symbols for one C atom and four H atoms, indicating the number of atoms in the molecule ( [link] ). The lines represent bonds that hold the atoms together. (A chemical bond is an attraction between atoms or ions that holds them together in a molecule or a crystal.) We will discuss chemical bonds and see how to predict the arrangement of atoms in a molecule later. For now, simply know that the lines are an indication of how the atoms are connected in a molecule. A ball-and-stick model shows the geometric arrangement of the atoms with atomic sizes not to scale, and a space-filling model shows the relative sizes of the atoms.
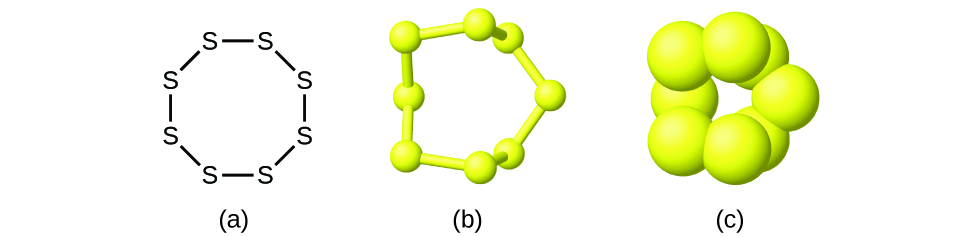
Although many elements consist of discrete, individual atoms, some exist as molecules made up of two or more atoms of the element chemically bonded together. For example, most samples of the elements hydrogen, oxygen, and nitrogen are composed of molecules that contain two atoms each (called diatomic molecules) and thus have the molecular formulas H 2 , O 2 , and N 2 , respectively. Other elements commonly found as diatomic molecules are fluorine (F 2 ), chlorine (Cl 2 ), bromine (Br 2 ), and iodine (I 2 ). The most common form of the element sulfur is composed of molecules that consist of eight atoms of sulfur; its molecular formula is S 8 ( [link] ).
It is important to note that a subscript following a symbol and a number in front of a symbol do not represent the same thing; for example, H 2 and 2H represent distinctly different species. H 2 is a molecular formula; it represents a diatomic molecule of hydrogen, consisting of two atoms of the element that are chemically bonded together. The expression 2H, on the other hand, indicates two separate hydrogen atoms that are not combined as a unit. The expression 2H 2 represents two molecules of diatomic hydrogen ( [link] ).

Notification Switch
Would you like to follow the 'Chemistry' conversation and receive update notifications?