| << Chapter < Page | Chapter >> Page > |
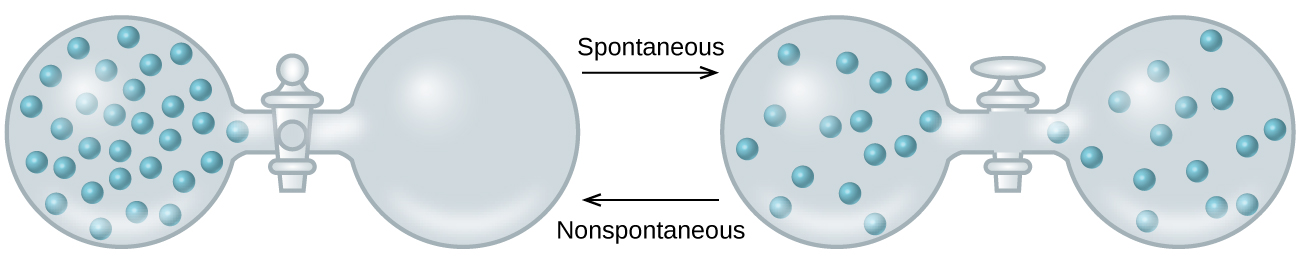
As we extend our discussion of thermodynamic concepts toward the objective of predicting spontaneity, consider now an isolated system consisting of two flasks connected with a closed valve. Initially there is an ideal gas on the left and a vacuum on the right ( [link] ). When the valve is opened, the gas spontaneously expands to fill both flasks. Recalling the definition of pressure-volume work from the chapter on thermochemistry, note that no work has been done because the pressure in a vacuum is zero.
Note as well that since the system is isolated, no heat has been exchanged with the surroundings ( q = 0). The first law of thermodynamics confirms that there has been no change in the system’s internal energy as a result of this process.
The spontaneity of this process is therefore not a consequence of any change in energy that accompanies the process. Instead, the driving force appears to be related to the greater, more uniform dispersal of matter that results when the gas is allowed to expand. Initially, the system was comprised of one flask containing matter and another flask containing nothing. After the spontaneous process took place, the matter was distributed both more widely (occupying twice its original volume) and more uniformly (present in equal amounts in each flask).
Now consider two objects at different temperatures: object X at temperature T X and object Y at temperature T Y , with T X > T Y ( [link] ). When these objects come into contact, heat spontaneously flows from the hotter object (X) to the colder one (Y). This corresponds to a loss of thermal energy by X and a gain of thermal energy by Y.
From the perspective of this two-object system, there was no net gain or loss of thermal energy, rather the available thermal energy was redistributed among the two objects. This spontaneous process resulted in a more uniform dispersal of energy .

As illustrated by the two processes described, an important factor in determining the spontaneity of a process is the extent to which it changes the dispersal or distribution of matter and/or energy. In each case, a spontaneous process took place that resulted in a more uniform distribution of matter or energy.
(a) A solid sublimes.
(b) A gas condenses.
(c) A drop of food coloring added to a glass of water forms a solution with uniform color.

(a) Sublimation is the conversion of a solid (relatively high density) to a gas (much lesser density). This process yields a much greater dispersal of matter, since the molecules will occupy a much greater volume after the solid-to-gas transition.
(b) Condensation is the conversion of a gas (relatively low density) to a liquid (much greater density). This process yields a much lesser dispersal of matter, since the molecules will occupy a much lesser volume after the solid-to-gas transition.
(c) The process in question is dilution. The food dye molecules initially occupy a much smaller volume (the drop of dye solution) than they occupy once the process is complete (in the full glass of water). The process therefore entails a greater dispersal of matter. The process may also yield a more uniform dispersal of matter, since the initial state of the system involves two regions of different dye concentrations (high in the drop, zero in the water), and the final state of the system contains a single dye concentration throughout.
This is also a dilution process, analogous to example (c). It entails both a greater and more uniform dispersal of matter as the compressed air in the canister is permitted to expand into the lower-pressure air of the room.
Chemical and physical processes have a natural tendency to occur in one direction under certain conditions. A spontaneous process occurs without the need for a continual input of energy from some external source, while a nonspontaneous process requires such. Systems undergoing a spontaneous process may or may not experience a gain or loss of energy, but they will experience a change in the way matter and/or energy is distributed within the system.
What is a spontaneous reaction?
A reaction has a natural tendency to occur and takes place without the continual input of energy from an external source.
What is a nonspontaneous reaction?
Indicate whether the following processes are spontaneous or nonspontaneous.
(a) Liquid water freezing at a temperature below its freezing point
(b) Liquid water freezing at a temperature above its freezing point
(c) The combustion of gasoline
(d) A ball thrown into the air
(e) A raindrop falling to the ground
(f) Iron rusting in a moist atmosphere
(a) spontaneous; (b) nonspontaneous; (c) spontaneous; (d) nonspontaneous; (e) spontaneous; (f) spontaneous
A helium-filled balloon spontaneously deflates overnight as He atoms diffuse through the wall of the balloon. Describe the redistribution of matter and/or energy that accompanies this process.
Many plastic materials are organic polymers that contain carbon and hydrogen. The oxidation of these plastics in air to form carbon dioxide and water is a spontaneous process; however, plastic materials tend to persist in the environment. Explain.
Although the oxidation of plastics is spontaneous, the rate of oxidation is very slow. Plastics are therefore kinetically stable and do not decompose appreciably even over relatively long periods of time.

Notification Switch
Would you like to follow the 'Chemistry' conversation and receive update notifications?