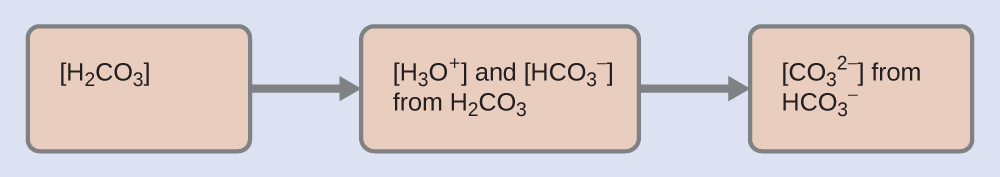Solution
As indicated by the ionization constants, H
2 CO
3 is a much stronger acid than
so H
2 CO
3 is the dominant producer of hydronium ion in solution. Thus there are two parts in the solution of this problem: (1) Using the customary four steps, we determine the concentration of H
3 O
+ and
produced by ionization of H
2 CO
3 . (2) Then we determine the concentration of
in a solution with the concentration of H
3 O
+ and
determined in (1). To summarize:
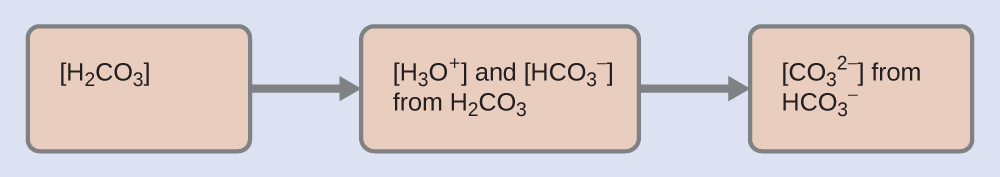
-
Determine the concentrations of
and
As for the ionization of any other weak acid:
An abbreviated table of changes and concentrations shows:
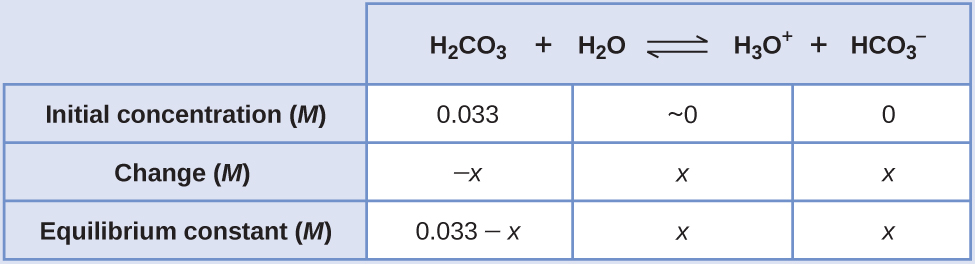
Substituting the equilibrium concentrations into the equilibrium gives us:
Solving the preceding equation making our standard assumptions gives:
Thus:
-
Determine the concentration of
in a solution at equilibrium with
and
both equal to 1.2
10
−4 M .
To summarize: In part 1 of this example, we found that the H
2 CO
3 in a 0.033-
M solution ionizes slightly and at equilibrium [H
2 CO
3 ] = 0.033
M ;
= 1.2
10
−4 ; and
In part 2, we determined that
Check your learning
The concentration of H
2 S in a saturated aqueous solution at room temperature is approximately 0.1
M . Calculate
[HS
− ], and [S
2− ] in the solution:
Answer:
[H
2 S] = 0.1
M ;
= [HS
− ] = 0.000094
M ; [S
2− ] = 1
10
−19
M
We note that the concentration of the sulfide ion is the same as
K
a2 . This is due to the fact that each subsequent dissociation occurs to a lesser degree (as acid gets weaker).
A
triprotic acid is an acid that has three dissociable protons that undergo stepwise ionization: Phosphoric acid is a typical example:
As with the diprotic acids, the differences in the ionization constants of these reactions tell us that in each successive step the degree of ionization is significantly weaker. This is a general characteristic of polyprotic acids and successive ionization constants often differ by a factor of about 10
5 to 10
6 .
This set of three dissociation reactions may appear to make calculations of equilibrium concentrations in a solution of H
3 PO
4 complicated. However, because the successive ionization constants differ by a factor of 10
5 to 10
6 , the calculations can be broken down into a series of parts similar to those for diprotic acids.
Polyprotic bases can accept more than one hydrogen ion in solution. The carbonate ion is an example of a
diprotic base , since it can accept up to two protons. Solutions of alkali metal carbonates are quite alkaline, due to the reactions: