| << Chapter < Page | Chapter >> Page > |
When you pour a glass of water, or fill a car with gasoline, you observe that water and gasoline flow freely. But when you pour syrup on pancakes or add oil to a car engine, you note that syrup and motor oil do not flow as readily. The viscosity of a liquid is a measure of its resistance to flow. Water, gasoline, and other liquids that flow freely have a low viscosity. Honey, syrup, motor oil, and other liquids that do not flow freely, like those shown in [link] , have higher viscosities. We can measure viscosity by measuring the rate at which a metal ball falls through a liquid (the ball falls more slowly through a more viscous liquid) or by measuring the rate at which a liquid flows through a narrow tube (more viscous liquids flow more slowly).
The IMFs between the molecules of a liquid, the size and shape of the molecules, and the temperature determine how easily a liquid flows. As [link] shows, the more structurally complex are the molecules in a liquid and the stronger the IMFs between them, the more difficult it is for them to move past each other and the greater is the viscosity of the liquid. As the temperature increases, the molecules move more rapidly and their kinetic energies are better able to overcome the forces that hold them together; thus, the viscosity of the liquid decreases.
| Viscosities of Common Substances at 25 °C | ||
|---|---|---|
| Substance | Formula | Viscosity (mPa·s) |
| water | H 2 O | 0.890 |
| mercury | Hg | 1.526 |
| ethanol | C 2 H 5 OH | 1.074 |
| octane | C 8 H 18 | 0.508 |
| ethylene glycol | CH 2 (OH)CH 2 (OH) | 16.1 |
| honey | variable | ~2,000–10,000 |
| motor oil | variable | ~50–500 |
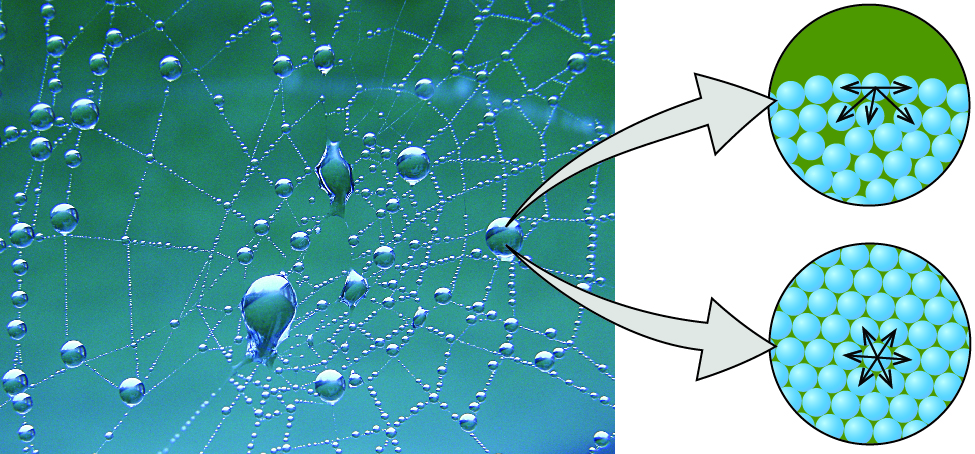
The various IMFs between identical molecules of a substance are examples of cohesive forces . The molecules within a liquid are surrounded by other molecules and are attracted equally in all directions by the cohesive forces within the liquid. However, the molecules on the surface of a liquid are attracted only by about one-half as many molecules. Because of the unbalanced molecular attractions on the surface molecules, liquids contract to form a shape that minimizes the number of molecules on the surface—that is, the shape with the minimum surface area. A small drop of liquid tends to assume a spherical shape, as shown in [link] , because in a sphere, the ratio of surface area to volume is at a minimum. Larger drops are more greatly affected by gravity, air resistance, surface interactions, and so on, and as a result, are less spherical.

Notification Switch
Would you like to follow the 'Chemistry' conversation and receive update notifications?