| << Chapter < Page | Chapter >> Page > |
(a) Multiply 0.6238 cm by 6.6 cm.
(b) Divide 421.23 g by 486 mL.
(a)
(b)
(b) Divide 55.8752 m by 56.53 s.
(a) 0.747 cm 2 (b) 0.9884 m/s
In the midst of all these technicalities, it is important to keep in mind the reason why we use significant figures and rounding rules—to correctly represent the certainty of the values we report and to ensure that a calculated result is not represented as being more certain than the least certain value used in the calculation.
1.034 g/mL
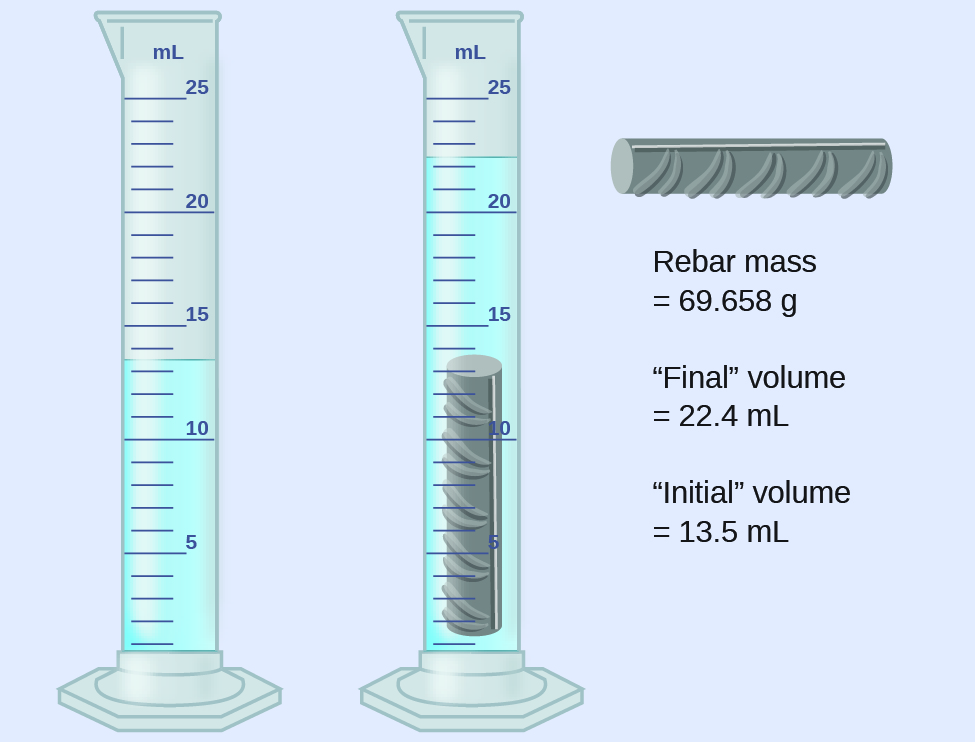
(a) Use these values to determine the density of this piece of rebar.
(b) Rebar is mostly iron. Does your result in (a) support this statement? How?
(rounded to the nearest 0.1 mL, per the rule for addition and subtraction)
The density is the mass-to-volume ratio:
(rounded to two significant figures, per the rule for multiplication and division)
From [link] , the density of iron is 7.9 g/cm 3 , very close to that of rebar, which lends some support to the fact that rebar is mostly iron.

(a) Use these values to determine the density of this material.
(b) Do you have any reasonable guesses as to the identity of this material? Explain your reasoning.
(a) 19 g/cm 3 ; (b) It is likely gold; the right appearance for gold and very close to the density given for gold in [link] .
Scientists typically make repeated measurements of a quantity to ensure the quality of their findings and to know both the precision and the accuracy of their results. Measurements are said to be precise if they yield very similar results when repeated in the same manner. A measurement is considered accurate if it yields a result that is very close to the true or accepted value. Precise values agree with each other; accurate values agree with a true value. These characterizations can be extended to other contexts, such as the results of an archery competition ( [link] ).

Notification Switch
Would you like to follow the 'Chemistry' conversation and receive update notifications?