| << Chapter < Page | Chapter >> Page > |
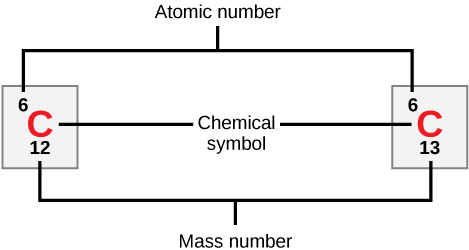
How many neutrons do carbon-12 and carbon-13 have, respectively?
Isotopes are different forms of an element that have the same number of protons but a different number of neutrons. Some elements—such as carbon, potassium, and uranium—have naturally occurring isotopes. Carbon-12 contains six protons, six neutrons, and six electrons; therefore, it has a mass number of 12 (six protons and six neutrons). Carbon-14 contains six protons, eight neutrons, and six electrons; its atomic mass is 14 (six protons and eight neutrons). These two alternate forms of carbon are isotopes. Some isotopes may emit neutrons, protons, and electrons, and attain a more stable atomic configuration (lower level of potential energy); these are radioactive isotopes, or radioisotopes . Radioactive decay (carbon-14 losing neutrons to eventually become carbon-12) describes the energy loss that occurs when an unstable atom’s nucleus releases radiation.
After approximately 5,730 years, half of the starting concentration of 14 C will have been converted back to 14 N. The time it takes for half of the original concentration of an isotope to decay back to its more stable form is called its half-life. Because the half-life of 14 C is long, it is used to date formerly living objects such as old bones or wood. Comparing the ratio of the 14 C concentration found in an object to the amount of 14 C detected in the atmosphere, the amount of the isotope that has not yet decayed can be determined. On the basis of this amount, the age of the material, such as the pygmy mammoth shown in [link] , can be calculated with accuracy if it is not much older than about 50,000 years. Other elements have isotopes with different half lives. For example, 40 K (potassium-40) has a half-life of 1.25 billion years, and 235 U (Uranium 235) has a half-life of about 700 million years. Through the use of radiometric dating, scientists can study the age of fossils or other remains of extinct organisms to understand how organisms have evolved from earlier species.


Notification Switch
Would you like to follow the 'Biology' conversation and receive update notifications?