| << Chapter < Page | Chapter >> Page > |
A colloid is a mixture that is somewhat like a heavy solution. The solute particles consist of tiny clumps of molecules large enough to make the liquid mixture opaque (because the particles are large enough to scatter light). Familiar examples of colloids are milk and cream. In the thyroid glands, the thyroid hormone is stored as a thick protein mixture also called a colloid.
A suspension is a liquid mixture in which a heavier substance is suspended temporarily in a liquid, but over time, settles out. This separation of particles from a suspension is called sedimentation. An example of sedimentation occurs in the blood test that establishes sedimentation rate, or sed rate. The test measures how quickly red blood cells in a test tube settle out of the watery portion of blood (known as plasma) over a set period of time. Rapid sedimentation of blood cells does not normally happen in the healthy body, but aspects of certain diseases can cause blood cells to clump together, and these heavy clumps of blood cells settle to the bottom of the test tube more quickly than do normal blood cells.
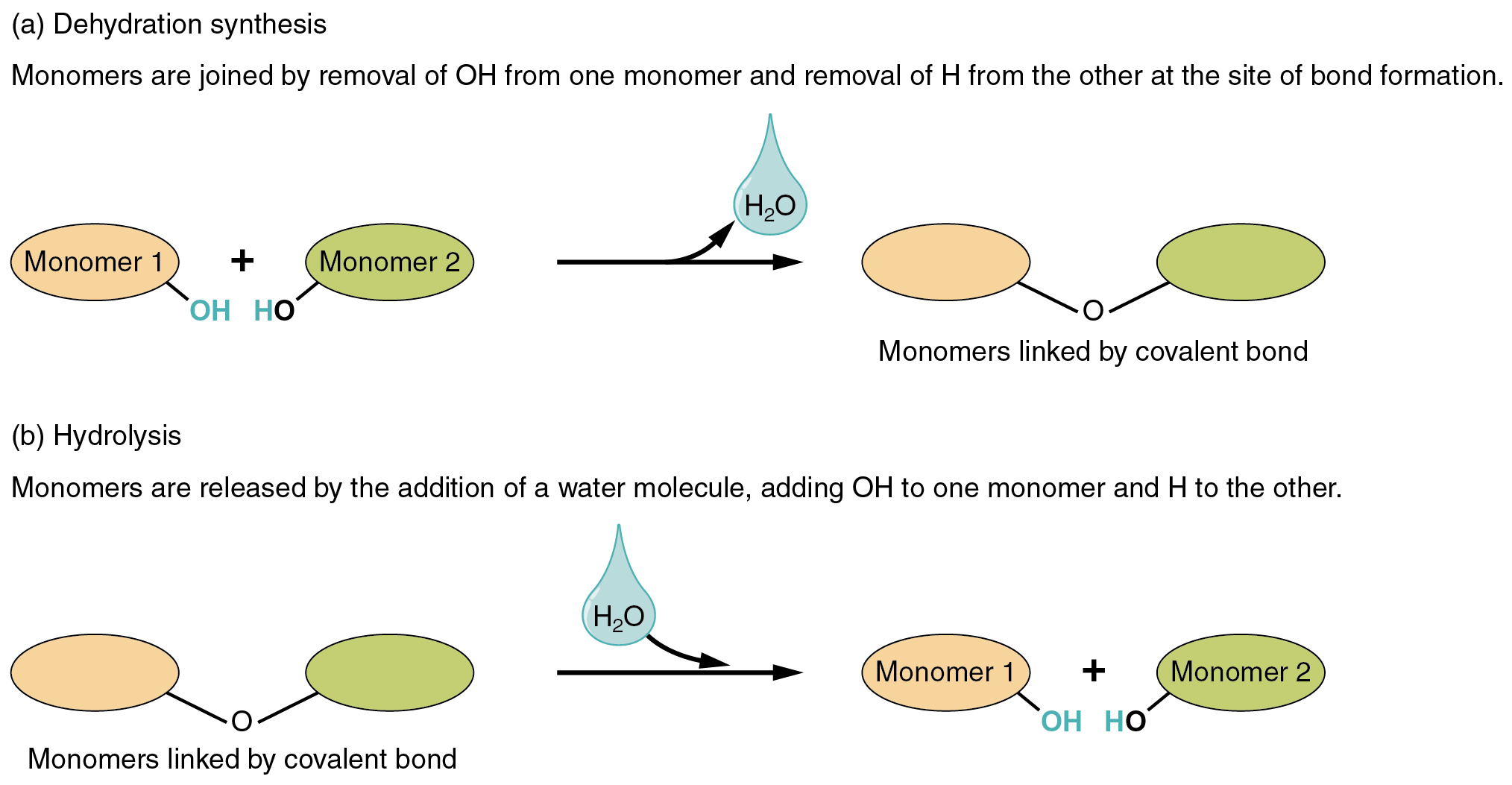
Two types of chemical reactions involve the creation or the consumption of water: dehydration synthesis and hydrolysis.
These reactions are reversible, and play an important role in the chemistry of organic compounds (which will be discussed shortly).
Recall that salts are formed when ions form ionic bonds. In these reactions, one atom gives up one or more electrons, and thus becomes positively charged, whereas the other accepts one or more electrons and becomes negatively charged. You can now define a salt as a substance that, when dissolved in water, dissociates into ions other than H + or OH – . This fact is important in distinguishing salts from acids and bases, discussed next.
A typical salt, NaCl, dissociates completely in water ( [link] ). The positive and negative regions on the water molecule (the hydrogen and oxygen ends respectively) attract the negative chloride and positive sodium ions, pulling them away from each other. Again, whereas nonpolar and polar covalently bonded compounds break apart into molecules in solution, salts dissociate into ions. These ions are electrolytes; they are capable of conducting an electrical current in solution. This property is critical to the function of ions in transmitting nerve impulses and prompting muscle contraction.

Notification Switch
Would you like to follow the 'Anatomy & Physiology' conversation and receive update notifications?