| << Chapter < Page | Chapter >> Page > |
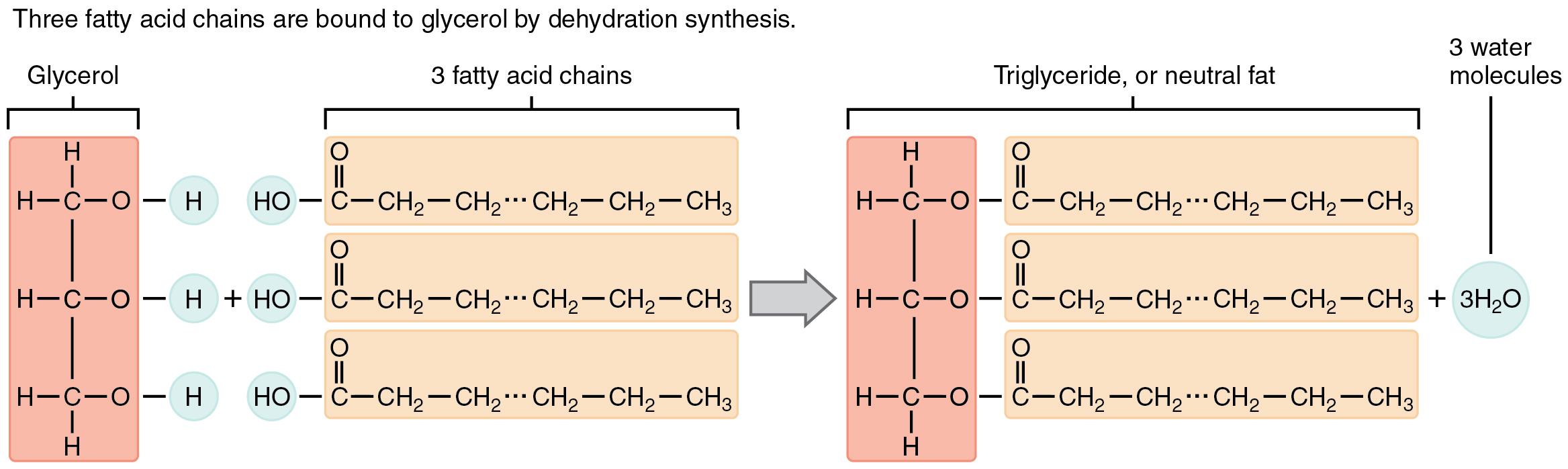
Triglycerides form via dehydration synthesis. Glycerol gives up hydrogen atoms from its hydroxyl groups at each bond, and the carboxyl group on each fatty acid chain gives up a hydroxyl group. A total of three water molecules are thereby released.
Fatty acid chains that have no double carbon bonds anywhere along their length and therefore contain the maximum number of hydrogen atoms are called saturated fatty acids. These straight, rigid chains pack tightly together and are solid or semi-solid at room temperature ( [link] a ). Butter and lard are examples, as is the fat found on a steak or in your own body. In contrast, fatty acids with one double carbon bond are kinked at that bond ( [link] b ). These monounsaturated fatty acids are therefore unable to pack together tightly, and are liquid at room temperature. Polyunsaturated fatty acids contain two or more double carbon bonds, and are also liquid at room temperature. Plant oils such as olive oil typically contain both mono- and polyunsaturated fatty acids.
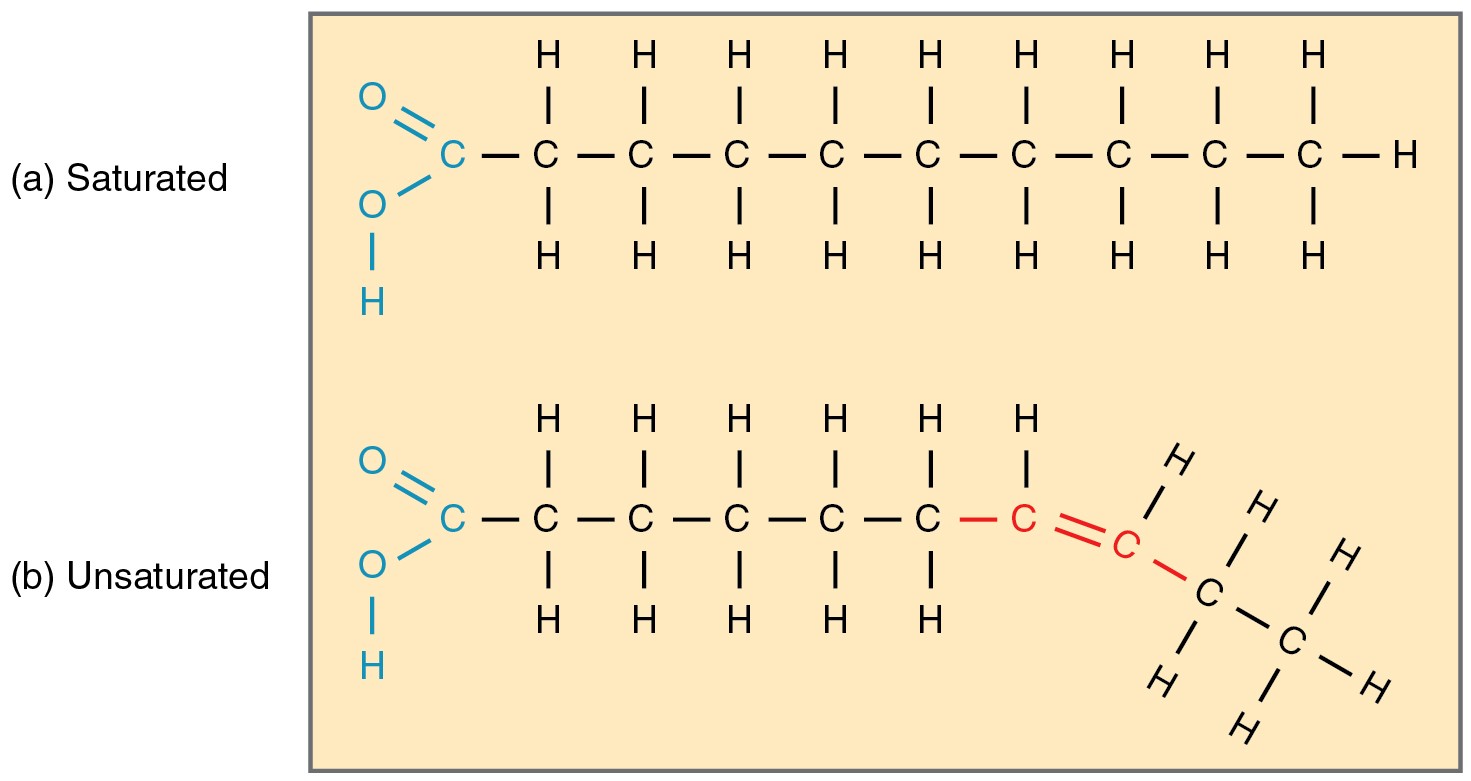
Whereas a diet high in saturated fatty acids increases the risk of heart disease, a diet high in unsaturated fatty acids is thought to reduce the risk. This is especially true for the omega-3 unsaturated fatty acids found in cold-water fish such as salmon. These fatty acids have their first double carbon bond at the third hydrocarbon from the methyl group (referred to as the omega end of the molecule).
Finally, trans fatty acids found in some processed foods, including some stick and tub margarines, are thought to be even more harmful to the heart and blood vessels than saturated fatty acids. Trans fats are created from unsaturated fatty acids (such as corn oil) when chemically treated to produce partially hydrogenated fats.
As a group, triglycerides are a major fuel source for the body. When you are resting or asleep, a majority of the energy used to keep you alive is derived from triglycerides stored in your fat (adipose) tissues. Triglycerides also fuel long, slow physical activity such as gardening or hiking, and contribute a modest percentage of energy for vigorous physical activity. Dietary fat also assists the absorption and transport of the nonpolar fat-soluble vitamins A, D, E, and K. Additionally, stored body fat protects and cushions the body’s bones and internal organs, and acts as insulation to retain body heat.
Fatty acids are also components of glycolipids, which are sugar-fat compounds found in the cell membrane. Lipoproteins are compounds in which the hydrophobic triglycerides are packaged in protein envelopes for transport in body fluids.

Notification Switch
Would you like to follow the 'Anatomy & Physiology' conversation and receive update notifications?